The translational canonical partition function Qtrs of an ideal monoatomic gas is given by: 3N 2T (alnQtrs ƏT A = Justify mathematically why the translational contribution to the molar constant-volume heat capacity is: 3 Cv=3Nk - Nk Derive an expression for pressure in terms of the canonical partition function Q, and then obtain an expression for the Gibbs energy in terms of the canonical partition function Q. ibl. (id 1) D n
The translational canonical partition function Qtrs of an ideal monoatomic gas is given by: 3N 2T (alnQtrs ƏT A = Justify mathematically why the translational contribution to the molar constant-volume heat capacity is: 3 Cv=3Nk - Nk Derive an expression for pressure in terms of the canonical partition function Q, and then obtain an expression for the Gibbs energy in terms of the canonical partition function Q. ibl. (id 1) D n
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter18: More Statistical Thermodynamics
Section: Chapter Questions
Problem 18.4E
Related questions
Question
Please answer all questions and please provide reasonings for using the certain equations.
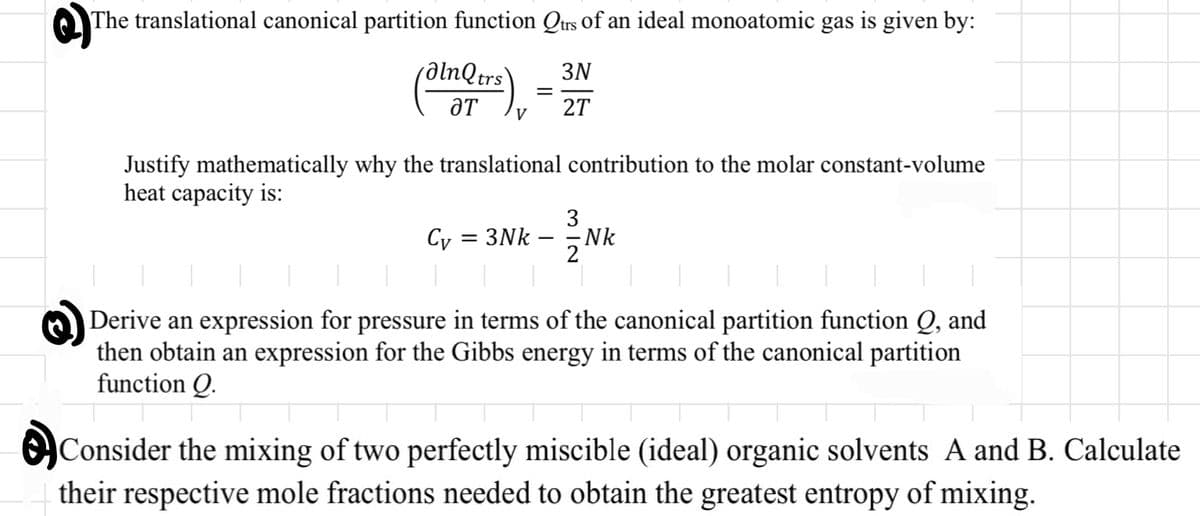
Transcribed Image Text:The translational canonical partition function Qtrs of an ideal monoatomic gas is given by:
3N
alnQtrs
ƏT
V
Cy
=
Justify mathematically why the translational contribution to the molar constant-volume
heat capacity is:
= 3Nk
27
312
Nk
Derive an expression for pressure in terms of the canonical partition function Q, and
then obtain an expression for the Gibbs energy in terms of the canonical partition
function Q.
Consider the mixing of two perfectly miscible (ideal) organic solvents A and B. Calculate
their respective mole fractions needed to obtain the greatest entropy of mixing.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,