thermometer -1 A sample of glass, which has a specific heat capacity of 0.670 J-g .°C is put into a calorimeter (see sketch at right) that insulated container contains 300.0 g of water. The glass sample starts off at 93.1 °C and the temperature of the water starts off at 21.0 °C. When the temperature of the water stops changing it's 23.2 °C. The pressure remains constant at 1 atm. water Calculate the mass of the glass sample. Be sure your answer is rounded to the correct number of significant digits. sample a calorimeter
thermometer -1 A sample of glass, which has a specific heat capacity of 0.670 J-g .°C is put into a calorimeter (see sketch at right) that insulated container contains 300.0 g of water. The glass sample starts off at 93.1 °C and the temperature of the water starts off at 21.0 °C. When the temperature of the water stops changing it's 23.2 °C. The pressure remains constant at 1 atm. water Calculate the mass of the glass sample. Be sure your answer is rounded to the correct number of significant digits. sample a calorimeter
Chemistry: An Atoms First Approach
2nd Edition
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Steven S. Zumdahl, Susan A. Zumdahl
Chapter7: Chemical Energy
Section: Chapter Questions
Problem 109AE: A sample of nickel is heated to 99.8C and placed in a coffee-cup calorimeter containing 150.0 g...
Related questions
Question
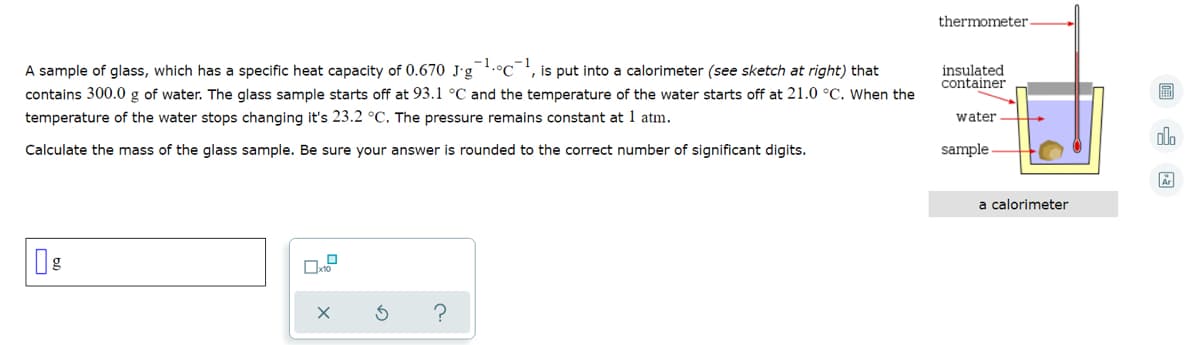
Transcribed Image Text:thermometer-
-1
is put into a calorimeter (see sketch at right) that
A sample of glass, which has a specific heat capacity of 0.670 J-g .°C
contains 300.0 g of water. The glass sample starts off at 93.1 °C and the temperature of the water starts off at 21.0 °C. When the
temperature of the water stops changing it's 23.2 °C. The pressure remains constant at 1 atm.
insulated
container
water
Calculate the mass of the glass sample. Be sure your answer is rounded to the correct number of significant digits.
sample
a calorimeter
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning