These are low energy electrons (-10-50 eV) generated from the collision between the incoming electrons and the loosely bonded outer electrons.
These are low energy electrons (-10-50 eV) generated from the collision between the incoming electrons and the loosely bonded outer electrons.
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 87E: Materials containing the elements Y, Ba, Cu, and O that are superconductors (electrical resistance...
Related questions
Question
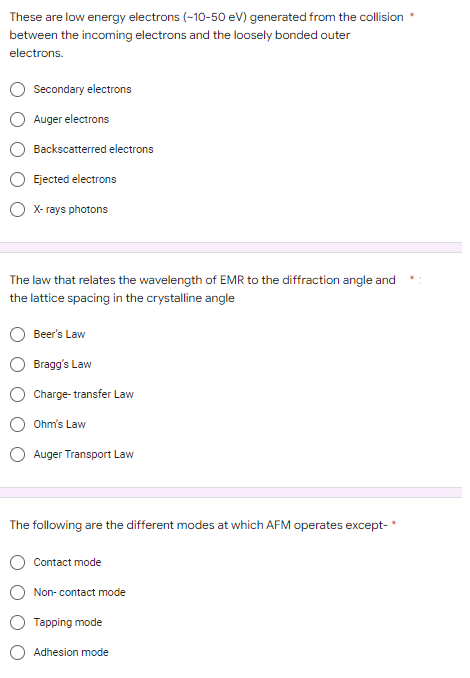
Transcribed Image Text:These are low energy electrons (-10-50 eV) generated from the collision
between the incoming electrons and the loosely bonded outer
electrons.
Secondary electrons
Auger electrons
Backscatterred electrons
Ejected electrons
X-rays photons
The law that relates the wavelength of EMR to the diffraction angle and
the lattice spacing in the crystalline angle
Beer's Law
Bragg's Law
Charge-transfer Law
Ohm's Law
Auger Transport Law
The following are the different modes at which AFM operates except-*
Contact mode
Non-contact mode
Tapping mode
Adhesion mode
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning