This activity focused on molecular (covalent) compounds, while an earlier activity addressed ionic compounds. Notice that the formulas for both types of compounds can look very similar, even though their names are quite different: Туре of Compound/Bonding Chemical Compound Name Formula Magnesium fluoride Copper(II) fluoride MGF2 Ionic CuF2 Ionic SF, Molecular (covalent) Sulfur difluoride NaBr Ionic Sodium bromide AuBr Ionic Gold(I) bromide IBr Molecular (covalent) Iodine monobromide Identify two differences between the names or formulas for ionic compounds versus those for binary molecular compounds. Also identify two similarities
This activity focused on molecular (covalent) compounds, while an earlier activity addressed ionic compounds. Notice that the formulas for both types of compounds can look very similar, even though their names are quite different: Туре of Compound/Bonding Chemical Compound Name Formula Magnesium fluoride Copper(II) fluoride MGF2 Ionic CuF2 Ionic SF, Molecular (covalent) Sulfur difluoride NaBr Ionic Sodium bromide AuBr Ionic Gold(I) bromide IBr Molecular (covalent) Iodine monobromide Identify two differences between the names or formulas for ionic compounds versus those for binary molecular compounds. Also identify two similarities
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter19: Complex Ions
Section: Chapter Questions
Problem 18QAP
Related questions
Question
solve asap with complete explanation within 30-40mins I will give you upvotes
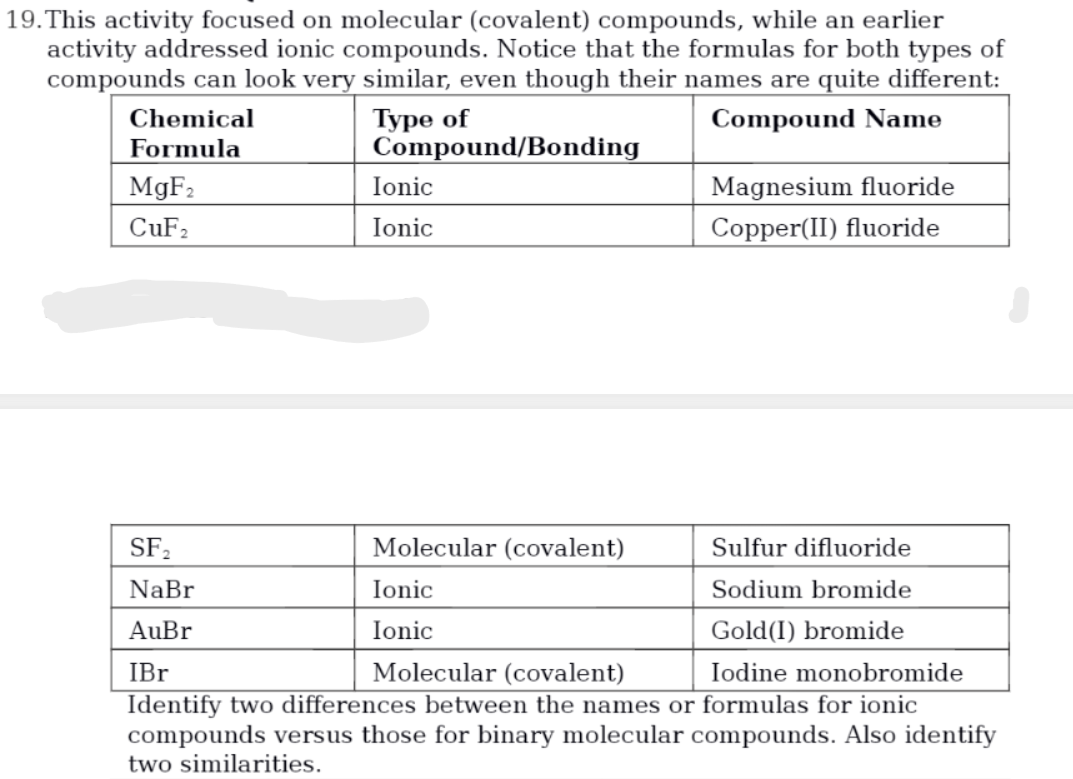
Transcribed Image Text:19.This activity focused on molecular (covalent) compounds, while an earlier
activity addressed ionic compounds. Notice that the formulas for both types of
compounds can look very similar, even though their names are quite different:
Туре of
Compound/Bonding
Chemical
Compound Name
Formula
MGF2
Ionic
Magnesium fluoride
CUF2
Ionic
Copper(II) fluoride
SF2
Molecular (covalent)
Sulfur difluoride
NaBr
Ionic
Sodium bromide
AuBr
Ionic
Gold(I) bromide
İBr
Molecular (covalent)
Iodine monobromide
Identify two differences between the names or formulas for ionic
compounds versus those for binary molecular compounds. Also identify
two similarities.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning