This exercise uses the radioactive decay model. The half-life of radium-226 is 1600 years. Suppose we have a 22-mg sample. (a) Find a function m(t) = mo2-t/h that models the mass remaining after t years. m(t) = (b) Find a function m(t) = moe¬rt that models the mass remaining after t years. (Round your r value to six decimal places.) m(t) =
This exercise uses the radioactive decay model. The half-life of radium-226 is 1600 years. Suppose we have a 22-mg sample. (a) Find a function m(t) = mo2-t/h that models the mass remaining after t years. m(t) = (b) Find a function m(t) = moe¬rt that models the mass remaining after t years. (Round your r value to six decimal places.) m(t) =
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter18: Nuclear Reactions
Section: Chapter Questions
Problem 81QAP
Related questions
Question
Transcribed Image Text:* 87%|
1 PM Thu Nov 12
A webassign.net
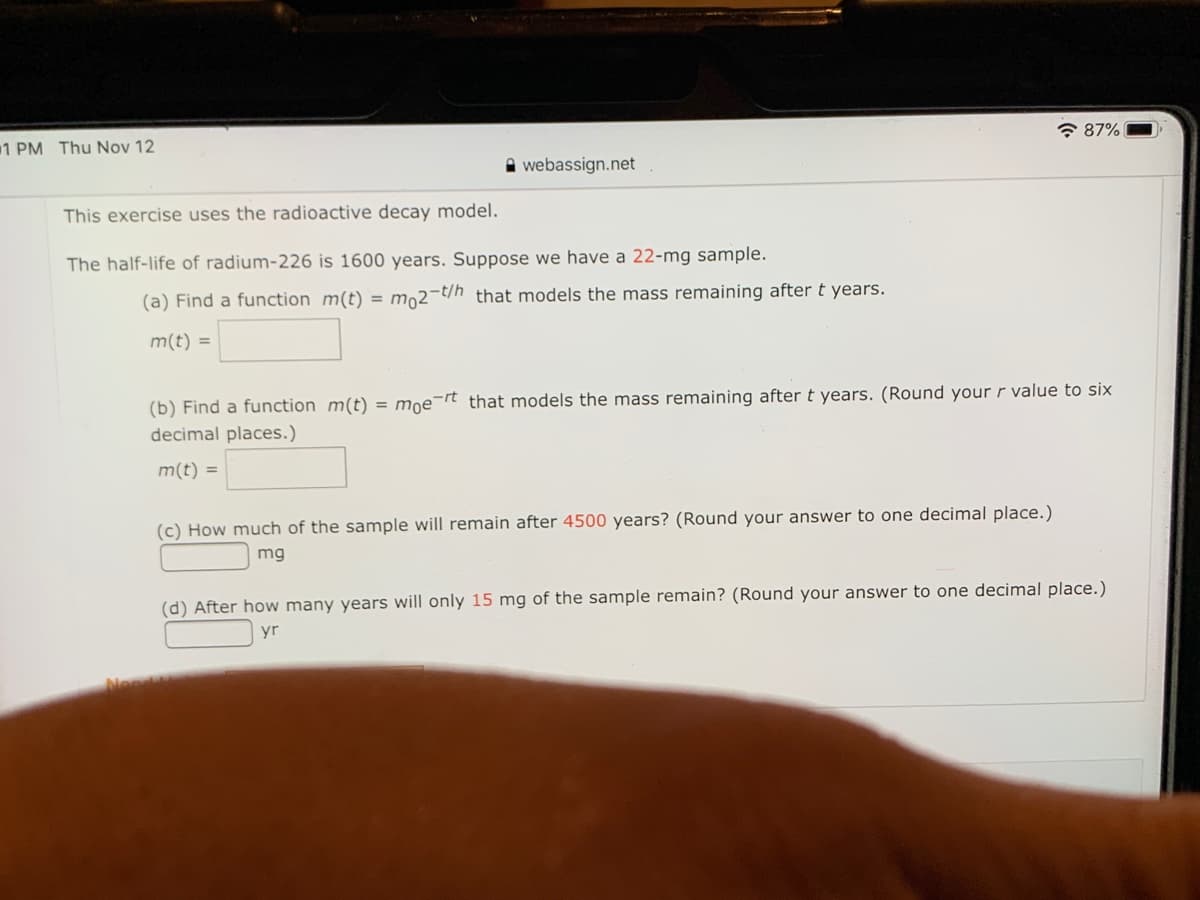
This exercise uses the radioactive decay model.
The half-life of radium-226 is 1600 years. Suppose we have a 22-mg sample.
(a) Find a function m(t) = mo2-t/h that models the mass remaining after t years.
m(t) =
(b) Find a function m(t) = moe¯rt that models the mass remaining after t years. (Round your r value to six
decimal places.)
m(t) =
(c) How much of the sample will remain after 4500 years? (Round your answer to one decimal place.)
mg
(d) After how many years will only 15 mg of the sample remain? (Round your answer to one decimal place.)
yr
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning