This question combines info from the chart AND your common sense (what you know about the world you live in). Which of the isotopes is most abundant on Earth? (1) Atomic (2) Element (3) Symbol (4) Mass (5) (6) % Abundance (or Radioactive (7) Half-life (if radioactive) Atomic Number Number, Mass Decay Mode) 0. (Neutron) 1.008665 10.4 min Hydrogen Deuterium 99.985% 0.015% H. 1.007825 2.014102 Tritium 3.016049 12.33 yr Helium Не 3.016029 0.000137% 99.999863% 4.002602 Lithium 7.5% 92.5% Li 6.015121 7.016003 EC, 7 100% 4 Beryllium Be 7.016928 53.29 days 9.012182 1-H (hydrogen with a mass number of 1) 2-H (hydrogen with a mass number of 2) 3-He (helium with a mass number of 3) 4-He (helium with a mass number of 4) 47 9
This question combines info from the chart AND your common sense (what you know about the world you live in). Which of the isotopes is most abundant on Earth? (1) Atomic (2) Element (3) Symbol (4) Mass (5) (6) % Abundance (or Radioactive (7) Half-life (if radioactive) Atomic Number Number, Mass Decay Mode) 0. (Neutron) 1.008665 10.4 min Hydrogen Deuterium 99.985% 0.015% H. 1.007825 2.014102 Tritium 3.016049 12.33 yr Helium Не 3.016029 0.000137% 99.999863% 4.002602 Lithium 7.5% 92.5% Li 6.015121 7.016003 EC, 7 100% 4 Beryllium Be 7.016928 53.29 days 9.012182 1-H (hydrogen with a mass number of 1) 2-H (hydrogen with a mass number of 2) 3-He (helium with a mass number of 3) 4-He (helium with a mass number of 4) 47 9
Living By Chemistry: First Edition Textbook
1st Edition
ISBN:9781559539418
Author:Angelica Stacy
Publisher:Angelica Stacy
ChapterU1: Alchemy: Matter, Atomic Structure, And Bonding
SectionU1.14: Isotopia: Stable And Radioactive Isotopes
Problem 13E
Related questions
Question
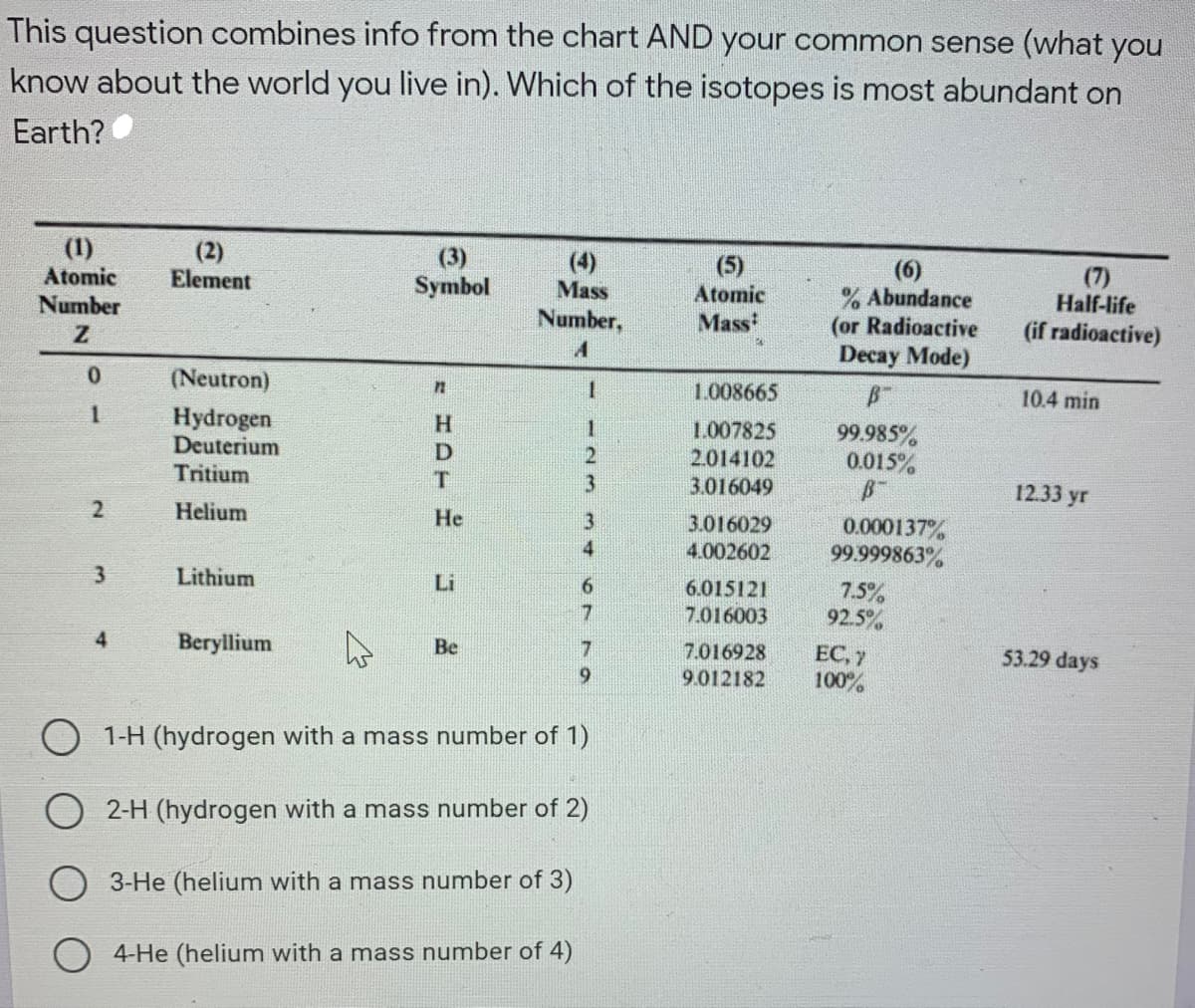
Transcribed Image Text:This question combines info from the chart AND your common sense (what you
know about the world you live in). Which of the isotopes is most abundant on
Earth?
(1)
Atomic
Number
(2)
Element
(3)
Symbol
(4)
Mass
(5)
(6)
% Abundance
(or Radioactive
(7)
Half-life
(if radioactive)
Atomic
Number,
Mass
Decay Mode)
0.
(Neutron)
1.008665
10.4 min
Hydrogen
Deuterium
Tritium
1.007825
2.014102
99.985%
0.015%
3.016049
12.33 yr
Helium
Не
3.016029
0.000137%
99.999863%
4.002602
Lithium
7.5%
92.5%
Li
6.015121
7.016003
EC, 7
100%
4
Beryllium
Be
7.016928
53.29 days
6.
9.012182
1-H (hydrogen with a mass number of 1)
2-H (hydrogen with a mass number of 2)
3-He (helium with a mass number of 3)
4-He (helium with a mass number of 4)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning