RADIATION DECAY .| g. I Name Symbol What is it? Charge What Chemical symbol for the element. Penetration Mass number A=p+n blocks it? depth (in air) Alpha a Helium nucleus 2 раper (2 protons & 2 neutrons) or Atomic number number of protons 8cm He Label which type of radiation would be blocked by the substance in the diagram below Beta High energy electron 3mm aluminum 1m or e Electromagnetic radiation Gamma Several meters of None infinite Paper aluminum (energy) lead or or concrete Y Lead/concrete Why does the beta particle have a negative charge? In the symbol for alpha, what does the 4 represent? Which has more mass--an alpha or beta particle? In the symbol for alpha, what does the 2 represent? Explain the zero & zero in the symbol for gamma: Why is the overall charge on the alpha particle +2?
RADIATION DECAY .| g. I Name Symbol What is it? Charge What Chemical symbol for the element. Penetration Mass number A=p+n blocks it? depth (in air) Alpha a Helium nucleus 2 раper (2 protons & 2 neutrons) or Atomic number number of protons 8cm He Label which type of radiation would be blocked by the substance in the diagram below Beta High energy electron 3mm aluminum 1m or e Electromagnetic radiation Gamma Several meters of None infinite Paper aluminum (energy) lead or or concrete Y Lead/concrete Why does the beta particle have a negative charge? In the symbol for alpha, what does the 4 represent? Which has more mass--an alpha or beta particle? In the symbol for alpha, what does the 2 represent? Explain the zero & zero in the symbol for gamma: Why is the overall charge on the alpha particle +2?
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter19: Nuclear Chemistry
Section: Chapter Questions
Problem 48AP
Related questions
Question
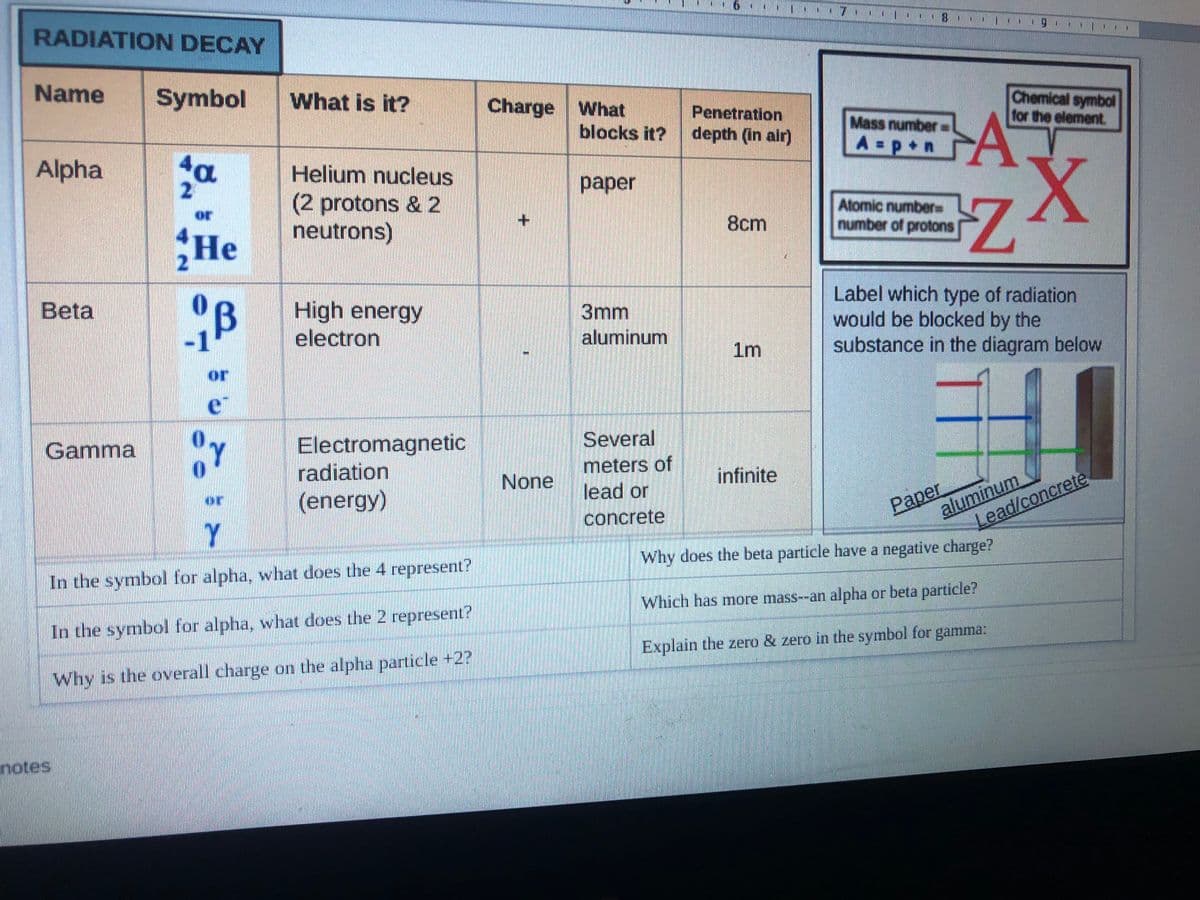
Transcribed Image Text:RADIATION DECAY
Name
Symbol
What is it?
Charge What
blocks it?
Penetration
depth (in air)
Chemical symbol
for the element.
Mass number
A p+n
Alpha
4a
Helium nucleus
раper
(2 protons & 2
neutrons)
Alomic numbere
number of protons
or
8cm
He
Label which type of radiation
would be blocked by the
substance in the diagram below
Beta
High energy
electron
3mm
aluminum
1m
or
e
Several
Electromagnetic
radiation
Gamma
meters of
lead or
infinite
Paper
aluminum
None
(energy)
or
concrete
Lead/concrete
Y
Why does the beta particle have a negative charge?
In the symbol for alpha, what does the 4 represent?
Which has more mass--an alpha or beta particle?
In the symbol for alpha, what does the 2 represent?
Explain the zero & zero in the symbol for gamma:
Why is the overall charge on the alpha particle +2?
notes
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning