Three gases (8.00 g of methane, CH4, 18.0 g of ethane, C₂H6, and an unknown amount of propane, C3H8) were added to the same 10.0-L container. At 23.0 °C, the total pressure in the container is 4.80 atm. Calculate the partial pressure of each gas in the container. Express the pressure values numerically in atmospheres, separated by commas. Enter the partial pressure of methane first, then ethane, then propane. ► View Available Hint(s) 15. ΑΣΦ Pmethane, Pethane, Ppropane 1.21,1.46, 1.23 ? atm
Three gases (8.00 g of methane, CH4, 18.0 g of ethane, C₂H6, and an unknown amount of propane, C3H8) were added to the same 10.0-L container. At 23.0 °C, the total pressure in the container is 4.80 atm. Calculate the partial pressure of each gas in the container. Express the pressure values numerically in atmospheres, separated by commas. Enter the partial pressure of methane first, then ethane, then propane. ► View Available Hint(s) 15. ΑΣΦ Pmethane, Pethane, Ppropane 1.21,1.46, 1.23 ? atm
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter5: Gases
Section: Chapter Questions
Problem 80QAP
Related questions
Question
100%
Please help with part a and b.
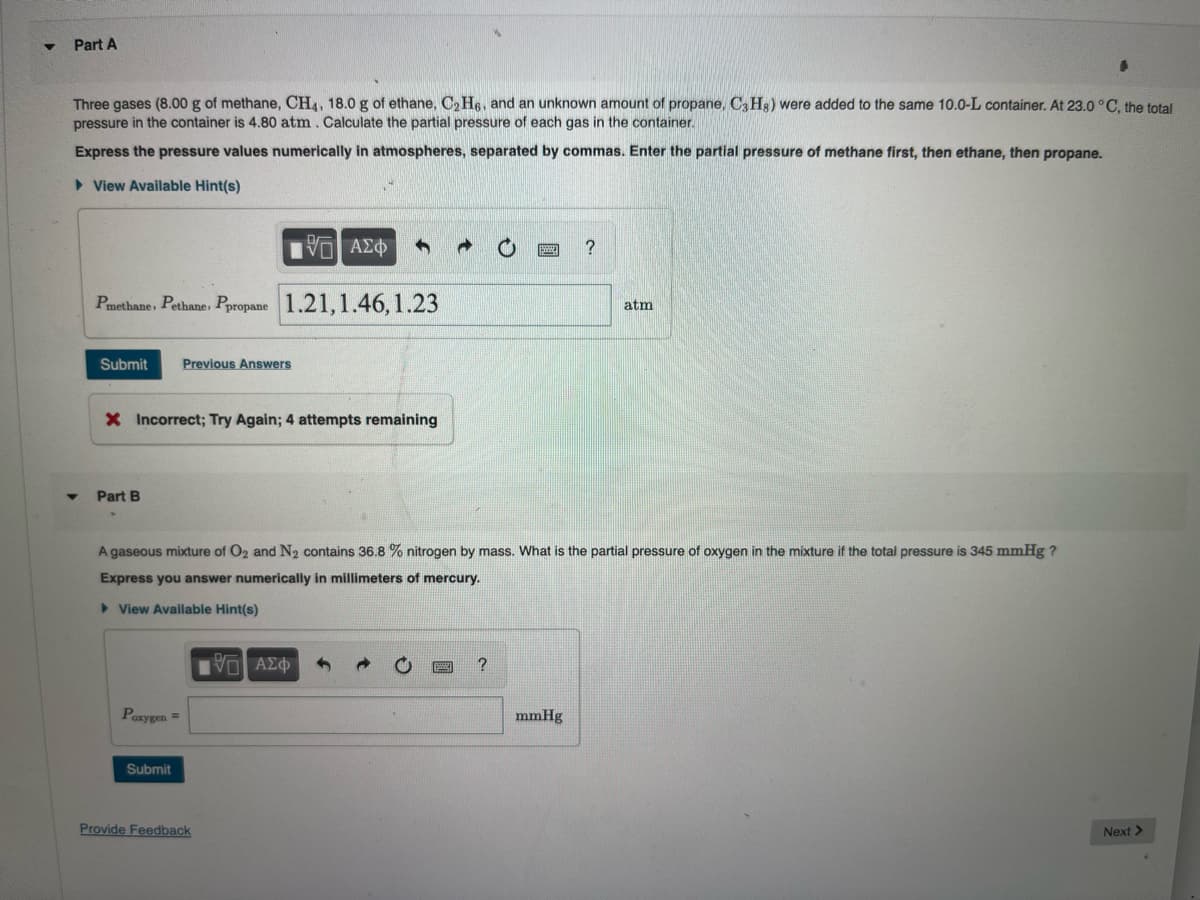
Transcribed Image Text:Part A
Three gases (8.00 g of methane, CH4, 18.0 g of ethane, C₂H6, and an unknown amount of propane, C3H8) were added to the same 10.0-L container. At 23.0 °C, the total
pressure in the container is 4.80 atm. Calculate the partial pressure of each gas in the container.
Express the pressure values numerically in atmospheres, separated by commas. Enter the partial pressure of methane first, then ethane, then propane.
► View Available Hint(s)
1951 ΑΣΦ 4
Pmethane, Pethane, Ppropane 1.21,1.46, 1.23
Submit Previous Answers
X Incorrect; Try Again; 4 attempts remaining
Part B
Poxygen =
A gaseous mixture of O2 and N₂ contains 36.8 % nitrogen by mass. What is the partial pressure of oxygen in the mixture if the total pressure is 345 mmHg ?
Express you answer numerically in millimeters of mercury.
View Available Hint(s)
Submit
Provide Feedback
IVE ΑΣΦ
?
?
mmHg
atm
•
Next >
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning