Ti³+ is to be generated in 0.10 M HCIO for coulometric reduction of azobenzene. TiO²+ + 2H+ + e Ti³+ + H₂O E = 0.100V 4 Ti³+ + C₂H₂N=NCH₂ + 4H₂O → Azobenzene At the counter electrode, water is oxidized, and O₂ is liberated at a pressure of 0.245 bar. Both electrodes are made of smooth Pt, and each has a total surface area of 1.00 cm². The rate of reduction of the azobenzene is 25.9 nmol/s, and the resistance of the solution between the generator electrodes is 46.0 2. Calculate the current density (A/m²) at the electrode surface. current density: 2 CH₂NH₂ + 4 TiO²+ + 4H+ Aniline Use Table 17-1 to estimate the overpotential for O₂ libertation. Table 17-1 Overpotential (V) for gas evolution at various current densities (A/m²) at 25°C 10 A/m² 100 A/m² 1 000 A/m² 10 000 A/m² Electrode Platinized Pt Smooth Pt H₂ 0.0154 0.024 0.721 0₂ H₂ 0₁₂ 0.398 0.0300 0.521 0.068 0.85 02₂ H₂ H₂ 0.0405 0.638 0.0483 0.288 1.28 0.676 0.766 1.49 A/m²
Ti³+ is to be generated in 0.10 M HCIO for coulometric reduction of azobenzene. TiO²+ + 2H+ + e Ti³+ + H₂O E = 0.100V 4 Ti³+ + C₂H₂N=NCH₂ + 4H₂O → Azobenzene At the counter electrode, water is oxidized, and O₂ is liberated at a pressure of 0.245 bar. Both electrodes are made of smooth Pt, and each has a total surface area of 1.00 cm². The rate of reduction of the azobenzene is 25.9 nmol/s, and the resistance of the solution between the generator electrodes is 46.0 2. Calculate the current density (A/m²) at the electrode surface. current density: 2 CH₂NH₂ + 4 TiO²+ + 4H+ Aniline Use Table 17-1 to estimate the overpotential for O₂ libertation. Table 17-1 Overpotential (V) for gas evolution at various current densities (A/m²) at 25°C 10 A/m² 100 A/m² 1 000 A/m² 10 000 A/m² Electrode Platinized Pt Smooth Pt H₂ 0.0154 0.024 0.721 0₂ H₂ 0₁₂ 0.398 0.0300 0.521 0.068 0.85 02₂ H₂ H₂ 0.0405 0.638 0.0483 0.288 1.28 0.676 0.766 1.49 A/m²
Chapter11: Dynamic Electrochemistry
Section: Chapter Questions
Problem 5P
Related questions
Question
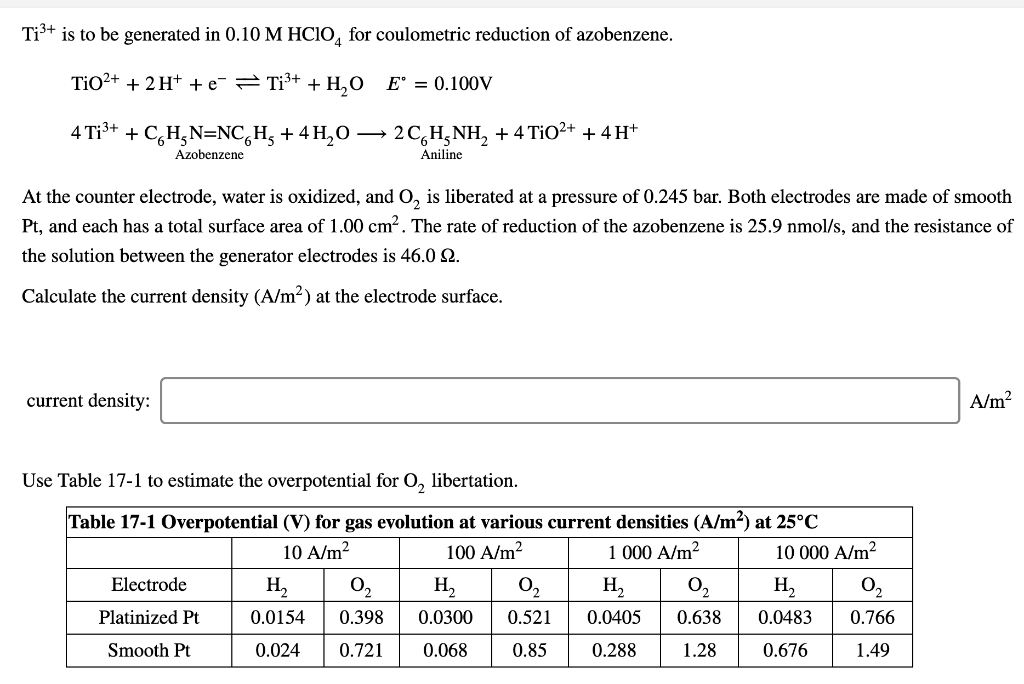
Transcribed Image Text:Ti³+ is to be generated in 0.10 M HCIO4 for coulometric reduction of azobenzene.
TiO²+ + 2H+ + e
Ti³+ + H₂O E° = 0.100V
4 Ti³+ + C₂H₂N=NC₂H₂ + 4H₂O → 2CH₂NH₂ + 4 TiO²+ + 4H+
Azobenzene
Aniline
At the counter electrode, water is oxidized, and O₂ is liberated at a pressure of 0.245 bar. Both electrodes are made of smooth
Pt, and each has a total surface area of 1.00 cm². The rate of reduction of the azobenzene is 25.9 nmol/s, and the resistance of
the solution between the generator electrodes is 46.0 92.
Calculate the current density (A/m²) at the electrode surface.
current density:
Use Table 17-1 to estimate the overpotential for O₂ libertation.
Table 17-1 Overpotential (V) for gas evolution at various current densities (A/m²) at 25°C
10 A/m²
100 A/m²
1 000 A/m²
10 000 A/m²
H₂
0₂
0.0405 0.638
0.288
1.28
Electrode
Platinized Pt
Smooth Pt
H₂
H₁₂
0₁₂
0.0154 0.398 0.0300 0.521
0.024 0.721
0.068
0.85
H₂
0.0483
0.676
0₂
0.766
1.49
A/m²
![overpotential:
Calculate the cathode potential (versus S.H.E.) assuming that [TiO²+]surface = [TiO²+]bulk = 0.0400 M and
[Ti³+] surface = 0.138 M.
E(cathode) =
Calculate the anode potential(versus S.H.E.). Refer to the table of standard reduction potentials as needed.
E(anode) =
What should be the applied voltage?
E =
V
V
V
V](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F8522cb1c-036f-4d7c-a3df-eb678cc22b38%2F1d419a63-6453-419c-90fa-7642617de939%2Fvei5ig_processed.png&w=3840&q=75)
Transcribed Image Text:overpotential:
Calculate the cathode potential (versus S.H.E.) assuming that [TiO²+]surface = [TiO²+]bulk = 0.0400 M and
[Ti³+] surface = 0.138 M.
E(cathode) =
Calculate the anode potential(versus S.H.E.). Refer to the table of standard reduction potentials as needed.
E(anode) =
What should be the applied voltage?
E =
V
V
V
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
