6. Calculate the average from the 2 experiments for each time point. Plot your data on a graph and calculate the rate (absorbance units minute ¹). Rate of the reaction based on your plotted values:
6. Calculate the average from the 2 experiments for each time point. Plot your data on a graph and calculate the rate (absorbance units minute ¹). Rate of the reaction based on your plotted values:
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 49RGQ: Fluoridation of city water supplies has been practiced in the United States for several decades. It...
Related questions
Question
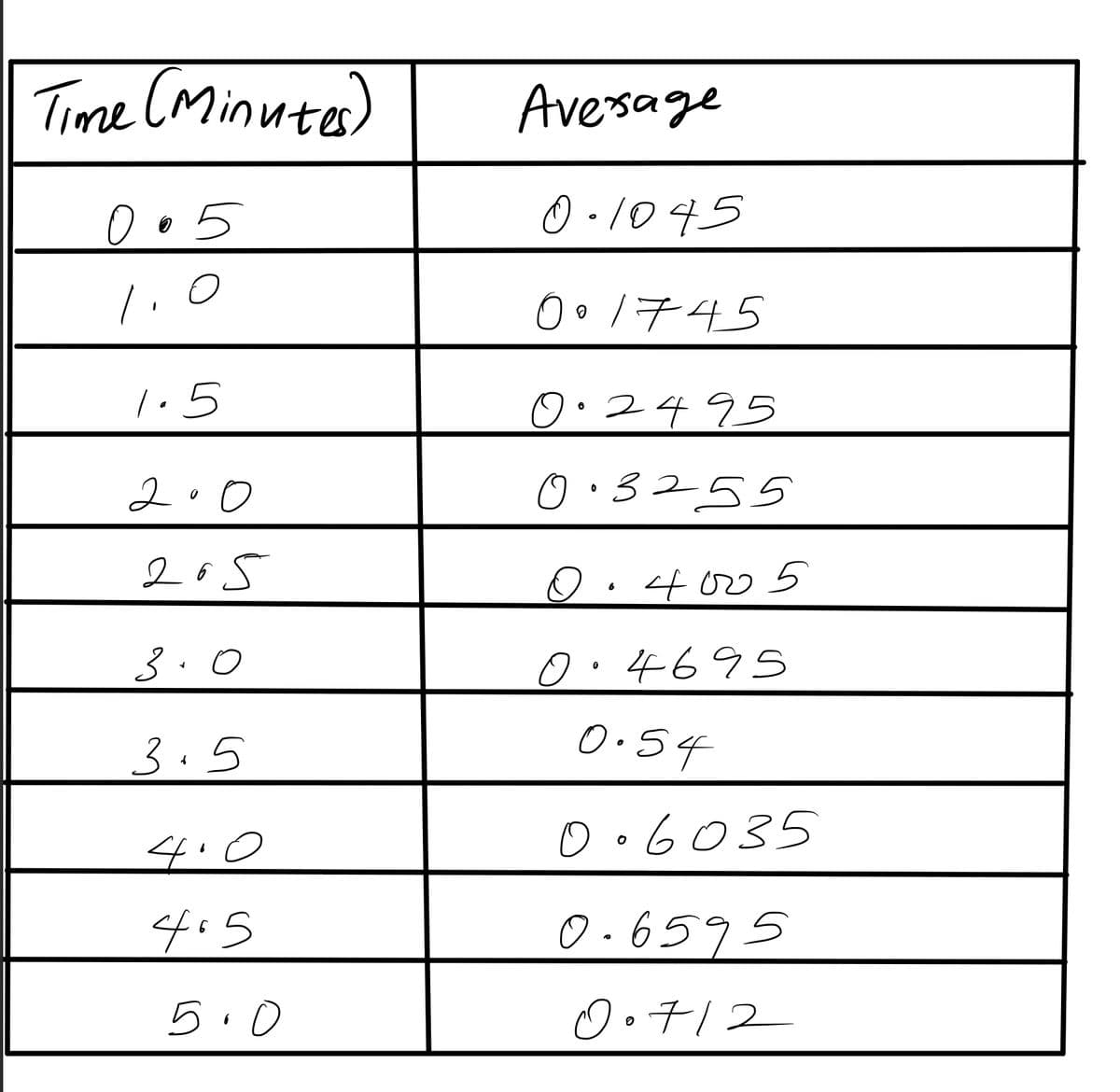
Transcribed Image Text:Time (Minutes)
0.5
1.0
1.5
200
205
3.0
3.5
4.0
4.5
6
5.0
Average
0.1045
001745
0.2495
0.3255
0.4005
0.4695
0.54
0.6035
0.6595
0.712

Transcribed Image Text:Apparatus:
●●●●
●●●
P1000 Gilson and blue gilson tips
3 disposable cuvettes
Spectrophotometer
parafilm
stop watch
tissue
Materials and Methods
Chemicals:
These chemicals are toxic, gloves and eye protection must be worn. All spills must be reported
to the lecturer or a demonstrator and cleaned up immediately. Students must wash their hands
before the laboratory is left.
50 mM sodium phosphate buffer, pH 7.5 (20ml)
Distilled water
2 mM 2-nitrophenyl-ß-D-galactopyranoside (ONP-Gal) (5ml)
2 mM 2-nitrophenyl-ß-glucopyranoside (ONP-Glu) (5ml)
2 units.ml-¹ B-galactosidase (from E. coli) (10ml)
Method:
1. Check that the spectrophotometer is on and set at 440 nm. If it is not set up correctly ASK for
help.
3. In the second cuvette add:
beslist elven
2. Fill the first cuvette with 3 ml distilled water and zero the spectrophotometer by placing the
cuvette in the machine in the correct orientation and pressing the BLUE button. If you follow the
instructions below you will not need to repeat this.
1 ml 50 mM sodium phosphate buffer, pH 7.5
1 ml Distilled water
0.5 ml 2-nitrophenyl-ß-D-galactopyranoside (ONP-Gal)
<pause here before moving to next step>
add 0.5ml ß-galactosidase to the cuvette WHEN YOU ARE READY TO START
THE CLOCK
Mix by covering the top of the cuvette with parafilm, ensure the outside of the cuvette is dry and
place in the correct orientation (bevelled sides to the left and right) in the spectrophotometer.
Press the GREEN button to start measuring the absorbance.
4. Take your first absorbance reading at 30 seconds and continue taking readings every 30
seconds for 5 minutes, record the reading in the form of a table (for table, see next page).
5. REPEAT the experiment starting at step 3.
6. Calculate the average from the 2 experiments for each time point. Plot your data on a graph
and calculate the rate (absorbance units minute).
Rate of the reaction based on your plotted values:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning