To answer this question, refer to the titration curve shown above and the points labelled A-G. Which of the following statements correctly describe the content of the solution being titrated at the different points during the titration? Select all that apply. There is a penalty for each missed or incorrect selection. UAt point A, there are more H30* ions than any other species present in solution (apart from water). At point B, there are equal amounts of A ions and HA molecules in solution. UAt point C, the concentration of H3O* ions in the solution is equal to the Ka value of the acid being titration. At point D, the solution in the beaker could be described as a buffer solution. UAt point E, the concentration of H3O* ions is the same as the concentration of OH" ions. At points F and G, the concentration of A ions in solution is approximately the same. UAt points F and G, the number of A ions in solution is approximately the same.
To answer this question, refer to the titration curve shown above and the points labelled A-G. Which of the following statements correctly describe the content of the solution being titrated at the different points during the titration? Select all that apply. There is a penalty for each missed or incorrect selection. UAt point A, there are more H30* ions than any other species present in solution (apart from water). At point B, there are equal amounts of A ions and HA molecules in solution. UAt point C, the concentration of H3O* ions in the solution is equal to the Ka value of the acid being titration. At point D, the solution in the beaker could be described as a buffer solution. UAt point E, the concentration of H3O* ions is the same as the concentration of OH" ions. At points F and G, the concentration of A ions in solution is approximately the same. UAt points F and G, the number of A ions in solution is approximately the same.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter15: Additional Aqueous Equilibria
Section15.2: Acid-base Titrations
Problem 15.6PSP: For the titration of 50.0 mL of 0.100-M HCl with 0.100-M NaOH, calculate the pH when these volumes...
Related questions
Question
4

Transcribed Image Text:To answer this question, refer to the titration curve shown above and the points
labelled A-G.
Which of the following statements correctly describe the content of the solution
being titrated at the different points during the titration?
Select all that apply. There is a penalty for each missed or incorrect selection.
UAt point A, there are more H30* ions than any other species present in solution
(apart from water).
UAt point B, there are equal amounts of A ions and HA molecules in solution.
UAt point C, the concentration of H3O* ions in the solution is equal to the Ka
value of the acid being titration.
At point D, the solution in the beaker could be described as a buffer solution.
At point E, the concentration of H3O* ions is the same as the concentration of
OH" ions.
UAt points F and G, the concentration of A ions in solution is approximately the
same.
UAt points F and G, the number of A ions in solution is approximately the same.
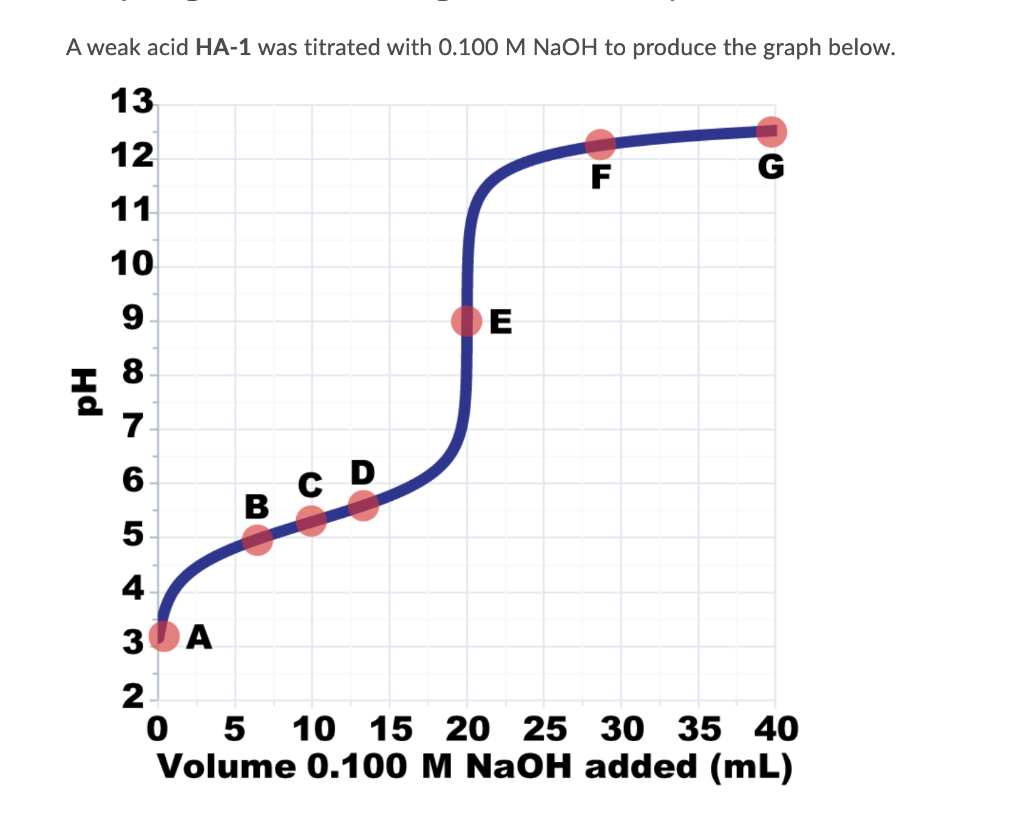
Transcribed Image Text:A weak acid HA-1 was titrated with 0.100 M NaOH to produce the graph below.
13
12
F
G
11
10
E
8
7
6
C D
B
4
30A
2
5
10
15 20 25 30 35 40
Volume 0.100 M NaOH added (mL)
Hd
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
