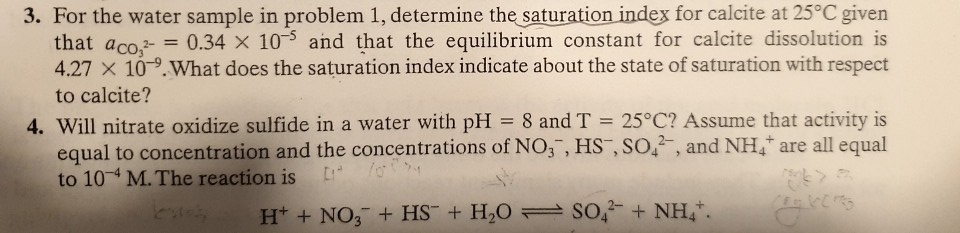
to calcite? 4. Will nitrate oxidize sulfide in a water with pH = 8 and T = 25°C? Assume that activity is equal to concentration and the concentrations of NO,, HS , SO,, and NH, are all equal
Q: 2. Aluminium sulphate (alum) is added to water with a pH of 7.2. Based on the reaction below,…
A: Formula:- PH=12 pKw - 12 pKb- 1210g C pKa of alum…
Q: For a Chromium-Nickel cell [Cr3+ + 3e- --> Cr (-0.73)] & [Ni2+ +2e- --> Ni (-0.23)] 1M for both…
A:
Q: com akEASSighment/takecovalentActivity.do?locator=assignment-take MISTRY [References] INTERACTIVE…
A: Here we have to calculate the time required for deposition of 1 gram of Ni from aqueous Ni^2+ with a…
Q: f Cl, and SiBr, were both liquids a 4
A:
Q: What are the advantages of dual alkali system?
A: The dual alkali scrubbing system is one of the systems which is used for the removal of sulfur oxide…
Q: 21.25 What is the difference between an active and an inactive electrode? Why are inactive…
A: Electrodes: In electrochemical cells, the electrodes are used as the conductors to supply the…
Q: What is the purpose of NaOH and 3% H2O2 in the separation of aluminum, iron (3+) and chromium ? Why…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Calculate the theoretical tendency of zinc to corrode (in volts) with evolution of hydrogen when…
A: Solution In the most typical use of the word, this suggests chemical science oxidisation of metal in…
Q: Analytical Chemistry II Why is carbon used as an electrode in arc and spark sources? Explain…
A: When a high voltage is applied to the material which is electrically insulating, it allows the flow…
Q: What is the time needed for a constant current of 0.100 A to deposit 0.500 g of Tl (I) [AW= 204.38…
A: Given: Mass of Tl to be formed = 0.500 g. Current passed = 0.100 A And the ion present in the…
Q: A concentration cell contains a 1.0 M solution of lanthanum nitrate [La(NOs))] in one compartment…
A: The solubility product constant represents the dissociation of solution in an aqueous solution,…
Q: Calculate AG" for the following reactions at 298 K ii. Cd + Fe²+ = Cd²+ +Fe [Cd'] = 0.01 M and [Fe²]…
A:
Q: To study the effects of a weakly acidic medium on the rate of corrosion of a metal alloy, a student…
A: First we would Calculate Kb value using Ka of acetic acid. Then first we write chemical equation…
Q: 5. A solution containing Pt4+ is electrolyzed with a current of 4.00 A. how long will it take to…
A: From the given molarity and volume, number of moles can be calculated. Then, the mass can be…
Q: What is the emf of the following cell at 25 °C? Ni(s) |Ni2*(1.00 M)||Fe3*(0.00500 M), Fe2*(0.010…
A:
Q: Calculate the activity coefficient of hydrogen in a 6M solution of a strong acid, given that…
A: We can use the following equations to calculate the activity coefficient of H+. pH = - log10 aH+…
Q: Consider the reaction of pyruvate with NADH and the standard reduction potentials, ?′°,E′°, of…
A:
Q: 6. Upon operation of the hollow cathode lamp at high current the intensity of the produced beam…
A: The operation of the hollow cathode lamp is in the following fashion: i) The positively charged…
Q: MgBr, CaO H,O K,O Cu-Zn alloy 0, CuCl, NO, TiO,
A: A chemical compound consists of two or more different elements which are bonded with each other…
Q: 1. Galculate the potential of a silver electrode following the addition of 10 ml. of 0.1 M of CaCl,…
A: The correct answer about electrochemical cell is given below
Q: A solution containing Pt* is electrolyzed with a current of 5.80 A. How long will it take to plate…
A: Moles of Pt4+ to be electrolyzed: = 99% × (0.20 L) × 0.025 M= 0.99 × (0.20) × 0.025 mol= 0.00495…
Q: Q2:- (A) electrode of oxygen and reference hydrogen electrode(H2) placed in an corrosion cell. (O2)…
A: Given the reference electrode is the hydrogen electrode, H2(g). Also given, O2(g) is reduced to…
Q: 1. Calculate the potential of a silver electrode following the addition of 10 ml of 0.1 M of CaCl to…
A:
Q: Aluminium sulphate (alum) is added to water with a pH of 7.2. Based on the reaction below,calculate…
A: The given reaction is the hydrolysis of the salt. The salt here is Al2 (SO4)3 which on hydrolysis…
Q: Iron corrodes to produce rust, Fe2O3, but other corrosionproducts that can form are Fe(O)(OH), iron…
A:
Q: Consider the formation of gibbsite from dissolved aluminum as depicted below: Al3+ + OH-…
A: Chemical reaction- It consists of reactant (starting material) and products (substances formed in…
Q: The Fe(3) in a 0.8202g sample was determined by coulometric reduction to fe(2) at the platinum…
A: The reaction is: Fe3++e-→Fe2+ It implies that: 96485 C is required to convert 1 mol of Fe3+ to Fe2+.…
Q: - How many coulombs required to obtain 54 gm of silver metal from its [Ag* = 108, charge of (e") =…
A:
Q: The sulfur from 4.00g steel is evolved as dihydrogen sulfide gas and titrated with 1.60 mL of…
A:
Q: A sample of pyrolusite weighs 0.5000 g. To this is added 0.6674 g of As2O3 and dilute acid. After…
A: NOTE
Q: 2. Aluminium sulphate (alum) is added to water with a pH of 7.2. Based on the reaction below,…
A: Dose of the alum = 30 mg/mL = 0.030 g/L The concentration of alum solution was prepared in Al+3…
Q: A 0.08 mM solution of potassium palmitate (K*Pal) is separated from a 0.120 mM solution of KCI by a…
A: Volume of potassium palmitate = 0.08 mM Volume of potassium chloride = 0.120 mM
Q: Calculate the copper activity in an aqueous 0.12 M CuCl2 solution.
A:
Q: Calculate the theoretical tendency of zinc to corrode (in volts) with evolution of hydrogen when…
A: Solution In the most typical use of the word, this suggests chemical science oxidisation of metal in…
Q: When Ca metal is placed in an aqueous solution of NaCl, the calcium slowly disappears and bubbles…
A: The activity of a metal depends on the electropositive nature of it. More the electropositive metal,…
Q: Calculate the theoretical potential at 25°C needed to initiate the deposition of copper from a…
A:
Q: The pNO, in a solution that is 750 ppm in Cu(NO,), and 3.0 x 103 M Zn(NO,), are ........ A.W Cu=63.5…
A: In order to calculate pNO3- we have to calculate the concentration of NO3- for each solution in…
Q: 9. What is the current that must be passed through solution AuCl3 for 200 second to precipitate 3g…
A: As per our guidelines, we are supposed to answer only one question. Kindly, repost other question as…
Q: Amounts of iodine dissolved in aqueous solution, Iz(aq), can be determined by titration with…
A: (a) The anode half-reaction for the oxidation of S2O32- to S4O62- is 2 S2O32- (aq) -->…
Q: Given the following electrode reactions: 2+ Co +e Co“ E'=1,92 V 3+ Co(NH,) , +e Co(NH,), E-0,06 a.…
A:
Q: Will acidic food coked in a cast iron skillet become FE2+, enriched because of a reaction between…
A:
Q: Calculate the time, in minutes, needed to plate 17.697 g of metal M at 43 Amps. The molar mass of…
A: this question is based on faraday second law: the mass of different ions liberated at the electrode…
Q: 2 In an electrolysis of copper sulphate between copper electrodes the total mass of copper deposited…
A:
Q: 2) Considering the redox potentials below: (a) Which Pu ion disproportionates? Write down the…
A: CONCEPT: Reduction: Gain of electron Oxidation: Loss of electron Reducing Agent: Species that reduce…
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

- Calculate the saturation indices of the solutions describe below and classify each as undersaturated, supersaturated, or in equilibrium. Saturation state with respect to: Anhydrite (CaSO4) [Ca2+] = 0.00002 mM [SO42-] = 25 mM “Amakinite” (Fe(OH)2) [Fe2+] = 0.1 mM; [OH-] = 0.0001 mM Siderite (FeCO3) [Fe2+] = 3 mM; [CO32-] = 10 mMGiven the solubility of ferrihydrite (Fe(OH)3) and goethite (FeOOH), with dissolution reactions shown below: [1] Fe(OH)3 3 H+ à Fe3+ + 3 H2O Ksp = 8.4 x 10^4 ∆ Gr0 = -28 KJ/mol Solubility of Fe(OH3) = 7.468 [2] FeOOH + 3 H+ à Fe3+ + 2 H2O Ksp = 8.8 x 10^-2 ∆ Gr0 = 6 KJ/mol Solubility of FeOOH = 0.353 QUESTION: [A] How much Fe3+ from ferrihydrite would dissolve at pH 2 ? [B] How much Fe3+ from ferrihydrite would dissolve at pH 7 ? [C] How much Fe3+ from goethite would dissolve at pH 2 ? [D] How much Fe3+ from goethite would dissolve at pH 7 ?Would you expect calcite to be appreciably soluble in a solution whose pH is maintained at 4? A solution at pH? A solution containing 0.1 m H2S? where might you find such solutions in nature?
- what wt. of limestone containing 9.57% Mg must be taken for analysis in order to precipitate of 0.551g Mg2P2O7? how many grams of Na2SO4 are required to ppte Ag2SO4 from 2.000t of AgNO3? a sample of magnetite (impure Fe3O4) weighing 0.5000g is fused with oxidizing flux and the ferric compound formed is eventually precipitated as ferric hydroxide and ignited to ferric oxide which weighs 0.4980calculate %Fe & %Fe2O31. What are the practical implications of alloying in carbon and alloy steels? 2. Make use of drawings of segments of the Fe-Cr phase diagram to illustrate the reason for the existence offerritic, martensitic and austenitic stainless steels by also noting the influence of specific alloying elementswhere-applicable. .A 0.9352g sample of ore containing Fe³+, Al³+ and Sr²+ was dissolved and made up to 500.00 mL. The analysis of metals was performed using complexation volumetry. Initially, an aliquot of 50.00 mL had its pH adjusted to 1.0 and titrated with a standard 0.03145 mol/L EDTA solution, requiring 6.95 mL to reach the end point. Subsequently, another 25.00 mL aliquot was buffered at pH=5 and titrated with the same EDTA solution, requiring 6.24 mL to reach the end point. Finally, a third aliquot of 25.00 mL was titrated at pH=11, requiring 11.10 mL of the same EDTA solution to complete the titration. Given the molar masses: Fe=55.845 g/mol; Al-26.982 g/mol and Sr-87.620 g/mol. a) Determine the percentage of each of the metals in the sample. b) Explain why the change in pH allows the determination of the three ions in this sample.
- If blast furnace slag is activated by NaOH, resulting in the formation of alkali-activated slag (AAS) – what kind of phases form in this system over time? How does the precipitated phase assemblage change with a change in the composition of the precursor slag? What differences exist in these formed phases in AAS systems when compared to blended systems such as OPC-Slag mixtures?A scientist was tasked to extract Fe from an aqueous suspension that contains 106 g of Fe2O3. The following steps detail the transformation of Fe2O3 (aq) to elemental iron: I. Enough sulfur trioxide gas was bubbled to Fe2O3 aqueous suspension to completely yield ferric sulfate. II. Then, 5.00 M nitric acid was added to ferric sulfate yielding an aqueous solution of ferric nitrate. III. Excess magnesium powder was added to the aqueous solution of ferric nitrate precipitating the solid iron ----- a. Write the balanced chemical reaction and the type of chemical reaction for [I], [II], [III]. Do not forget to indicate the states of the reactants and products (s, l, g).(CHOICES: COMBINATION, SINGLE DISPLACEMENT, DOUBLE DISPLACEMENT) b. What is the final mass of iron? Express final answers in 3 significant figures. c. High-concentration HCl is supposed to be added at the last part of the procedure. Briefly state its purpose.An unknown salt X reacts with hot conc. H2SO4 to produce a brown coloured gas which intensifies on addition on copper turnings. On adding dilute ferrous sulphate solution to an aqueous solution of X and then carefully adding conc. H2SO4along the sides of the test tube, a brown complex Y is formed at the interface between the solution and H2SO4. Identify X andY and write the chemical equation involved in the reaction.
- An exhausted zeolite softener was regenerated by passing 100 litres, of NaCl. Solution containing 150 gm per lit. of NaCl. How many lit. of a sample of H2O of hardness 300 ppm can be softened by this softener? (Given at wts. for C = 12, O = 16, Na = 23, CI = 35.5, Ca = 40).An unknown salt X reacts with hot conc. H2SO4 to produce a brown coloured gas which intensifies on addition on copper turnings. On adding dilute ferrous sulphate solution to an aqueous solution of X and then carefully adding conc. H2 SO4 along the sides of the test tube, a brown complex Y is formed at the interface between the solution and H2 SO4. Identify X and Y and write the chemical equation involved in the reaction.Compare the solubility of ferrihydrite (Fe(OH)3) and goethite (FeOOH), with dissolution reactions shown below: Fe(OH)3 + 3H+ <-> Fe3+ + 3H2O FeOOH + 3H+ <-> Fe3+ + 2H2O How much Fe3+ from ferrihydrite would dissolve at pH 2 and 7? How much Fe3+ from goethite would dissolve at pH 2 and 7?

