To carry out a particular reaction, you determine that you need 0.0500 moles of ammonium chloride. What volume of the solution described above will you need to complete the reaction without any leftover NH4CI? 0.54585 mL of solution
To carry out a particular reaction, you determine that you need 0.0500 moles of ammonium chloride. What volume of the solution described above will you need to complete the reaction without any leftover NH4CI? 0.54585 mL of solution
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 35CR
Related questions
Question
Answer Part 3
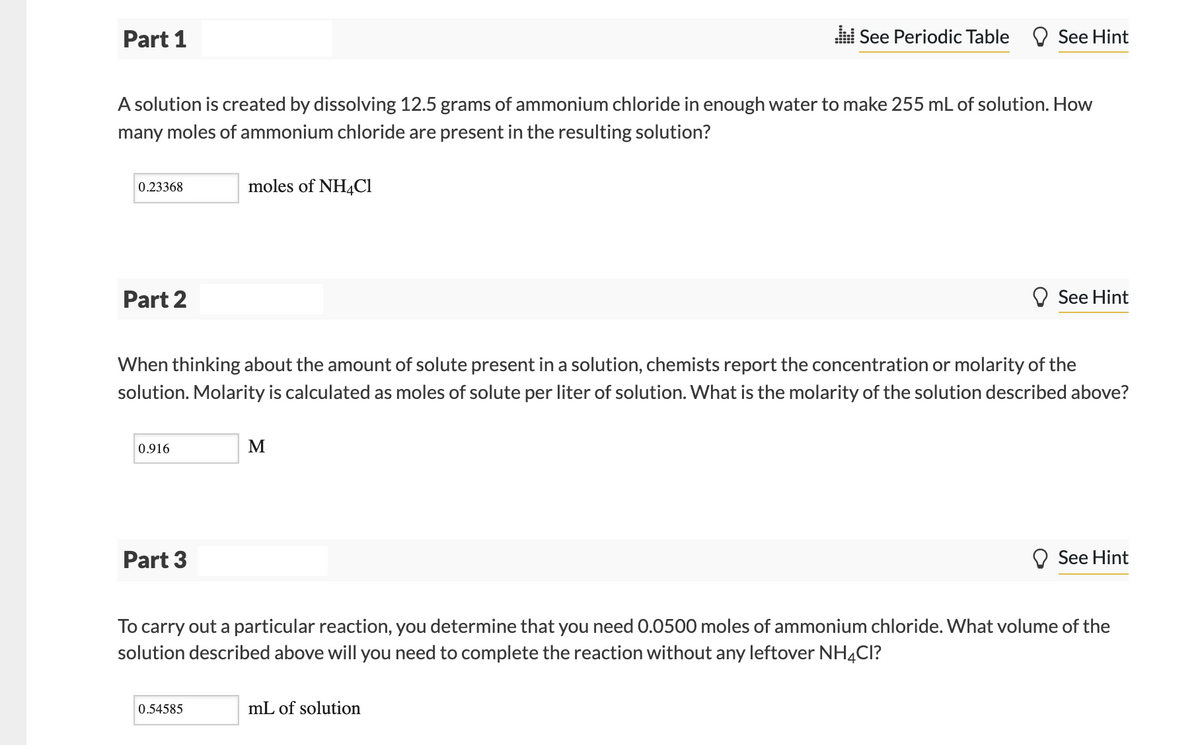
Transcribed Image Text:Part 1
l See Periodic Table O See Hint
A solution is created by dissolving 12.5 grams of ammonium chloride in enough water to make 255 mL of solution. How
many moles of ammonium chloride are present in the resulting solution?
0.23368
moles of NH4C1
Part 2
See Hint
When thinking about the amount of solute present in a solution, chemists report the concentration or molarity of the
solution. Molarity is calculated as moles of solute per liter of solution. What is the molarity of the solution described above?
0.916
M
Part 3
See Hint
To carry out a particular reaction, you determine that you need 0.0500 moles of ammonium chloride. What volume of the
solution described above will you need to complete the reaction without any leftover NH4CI?
0.54585
mL of solution
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
