To test a spectrophotometer's accuracy, a prepared and analyzed. This solution has 350.0 nm. Several aliquots of the solution values. 0.638 0.640 0.639 0.640 0.639 0.638 e any significant difference between the e at a 99.9% confidence level? s because tcal>tcritical
To test a spectrophotometer's accuracy, a prepared and analyzed. This solution has 350.0 nm. Several aliquots of the solution values. 0.638 0.640 0.639 0.640 0.639 0.638 e any significant difference between the e at a 99.9% confidence level? s because tcal>tcritical
Physics for Scientists and Engineers, Technology Update (No access codes included)
9th Edition
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Raymond A. Serway, John W. Jewett
Chapter34: Electromagnetic Waves
Section: Chapter Questions
Problem 34.7P: Suppose you are located 180 in from a radio transmitter. (a) How many wavelengths are you from the...
Related questions
Question
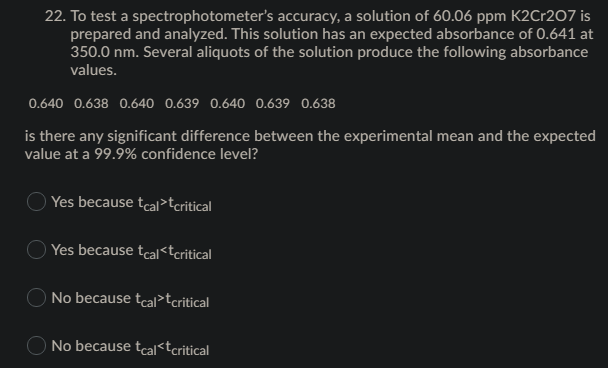
Transcribed Image Text:22. To test a spectrophotometer's accuracy, a solution of 60.06 ppm K2Cr2O7 is
prepared and analyzed. This solution has an expected absorbance of 0.641 at
350.0 nm. Several aliquots of the solution produce the following absorbance
values.
0.640 0.638 0.640 0.639 0.640 0.639 0.638
is there any significant difference between the experimental mean and the expected
value at a 99.9% confidence level?
Yes because tcal>tcritical
Yes because tcal<tcritical
No because tcal>tcritical
No because tcal<tcritical
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

Physics for Scientists and Engineers, Technology …
Physics
ISBN:
9781305116399
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning


University Physics Volume 3
Physics
ISBN:
9781938168185
Author:
William Moebs, Jeff Sanny
Publisher:
OpenStax

College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College

Principles of Physics: A Calculus-Based Text
Physics
ISBN:
9781133104261
Author:
Raymond A. Serway, John W. Jewett
Publisher:
Cengage Learning

College Physics
Physics
ISBN:
9781285737027
Author:
Raymond A. Serway, Chris Vuille
Publisher:
Cengage Learning