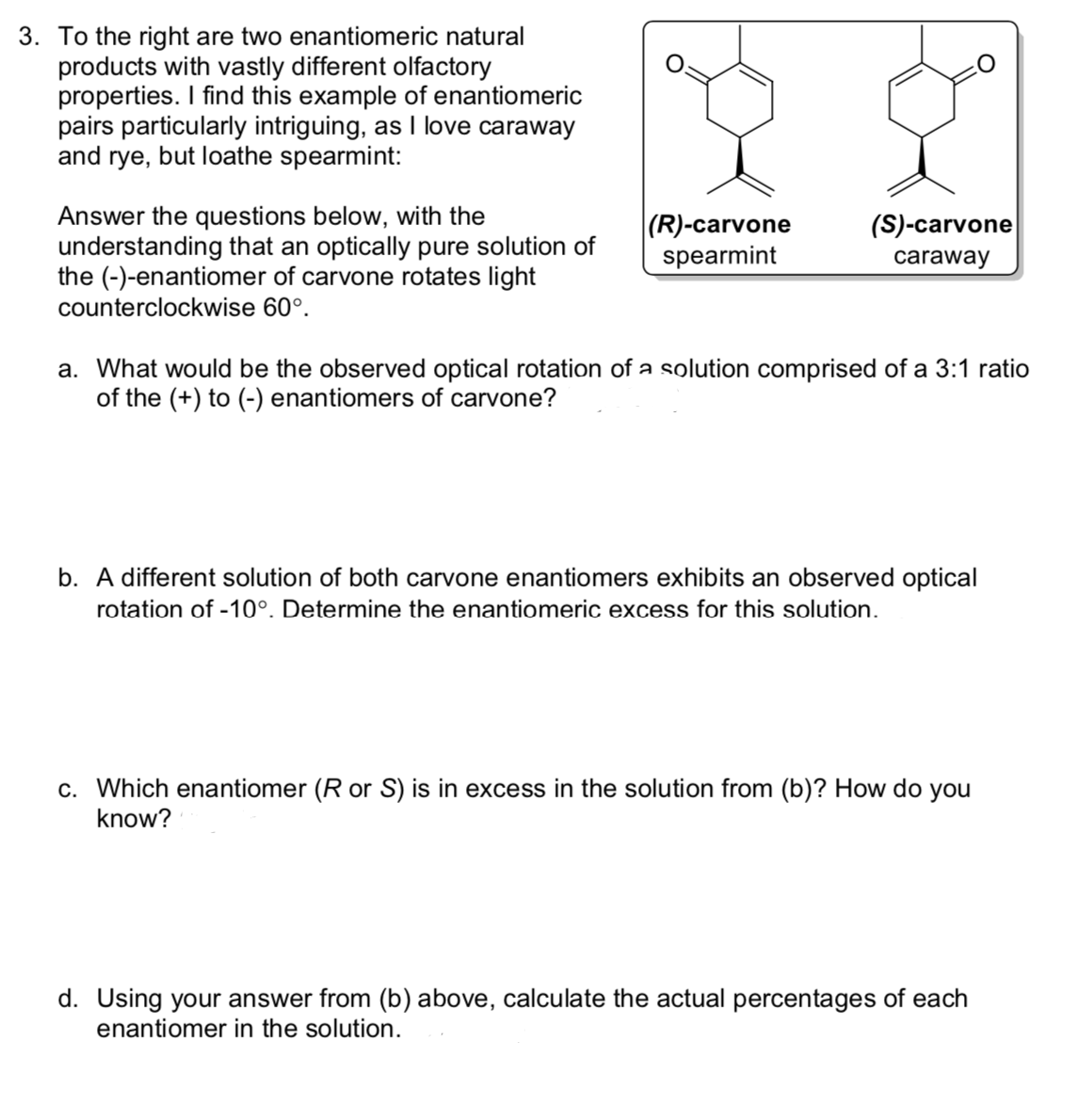
To the right are two enantiomeric natural products with vastly different olfactory properties. I find this example of enantiomeric pairs particularly intriguing, as I love caraway and rye but loathe searment: Answer the questions below with the understanding that an optically pure solution of the (-)-enantiomer of carvone rotates light counterclockwise 60*. a. what would be the observed optical rotation of a solution comprised of a 3:1 ratio of the (+) to (-) enantiomers of carvone? b. a different solution of both carvone enantiomers exhibits an observed optical rotation of -10*. determine the enantiomeric excess for this solution. c. which enantiomer (R or S) is in excess in the solution from (b)? how do you know? d. using your answer (b) above, calculate the actual percentages of each enantiomer in the solution.
To the right are two enantiomeric natural products with vastly different olfactory properties. I find this example of enantiomeric pairs particularly intriguing, as I love caraway and rye but loathe searment:
Answer the questions below with the understanding that an optically pure solution of the (-)-enantiomer of carvone rotates light counterclockwise 60*.
a. what would be the observed optical rotation of a solution comprised of a 3:1 ratio of the (+) to (-) enantiomers of carvone?
b. a different solution of both carvone enantiomers exhibits an observed optical rotation of -10*. determine the enantiomeric excess for this solution.
c. which enantiomer (R or S) is in excess in the solution from (b)? how do you know?
d. using your answer (b) above, calculate the actual percentages of each enantiomer in the solution.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images




