Trial Burette Reading @Start (MI) Burette Reading @End (MI) 1 2 0.00 11.30 11.30 22.60 3 22.60 33.80 Standard Used: 0.25 M Ca(OH)2 Volume Of Standard Used (MI) ? ? ?
Trial Burette Reading @Start (MI) Burette Reading @End (MI) 1 2 0.00 11.30 11.30 22.60 3 22.60 33.80 Standard Used: 0.25 M Ca(OH)2 Volume Of Standard Used (MI) ? ? ?
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.10QAP
Related questions
Question
100%
A titration was done to determine the concentration of a sample of HCl. The volume of the acid sample used in each titration trial was 10.00 mL. The following data also was collected:
a. Write the balanced neutralization reaction equation for this titration.
b. Write a brief procedure that would have been used to carry out the titration. Just include the key steps (terms and measurements)
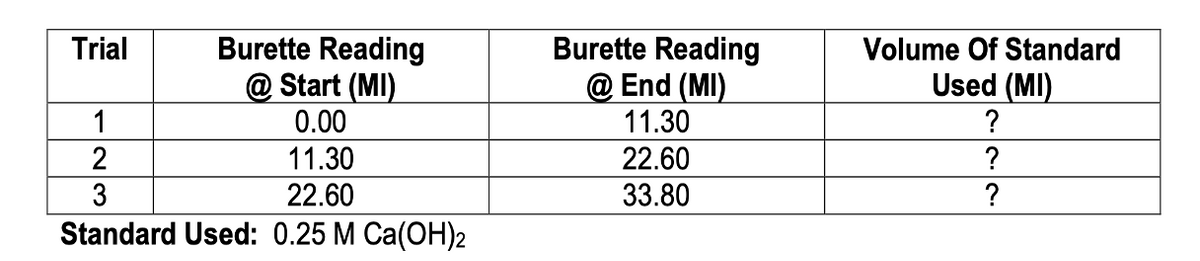
Transcribed Image Text:Trial
Burette Reading
@Start (MI)
Burette Reading
@End (MI)
1
2
0.00
11.30
11.30
22.60
3
22.60
33.80
Standard Used: 0.25 M Ca(OH)2
Volume Of Standard
Used (MI)
?
?
?
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
