Triphenylmethyl (trityl) groups are often used for the protection of alcohols because they are easily removed, as shown below. Think about the likely mechanism of this hydrolysis and then predict where the isotopic label would appear in the products if 18OH₂ was used. R hot Ph Ph -Ph H₂O EtOH LOH In the Ph,COH In the ROH In both the Ph,COH and the ROH In neither the Ph,COH nor the ROH + Ph offen Ph Ph HO
Triphenylmethyl (trityl) groups are often used for the protection of alcohols because they are easily removed, as shown below. Think about the likely mechanism of this hydrolysis and then predict where the isotopic label would appear in the products if 18OH₂ was used. R hot Ph Ph -Ph H₂O EtOH LOH In the Ph,COH In the ROH In both the Ph,COH and the ROH In neither the Ph,COH nor the ROH + Ph offen Ph Ph HO
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter14: Mass Spectrometry
Section: Chapter Questions
Problem 14.13P
Related questions
Question
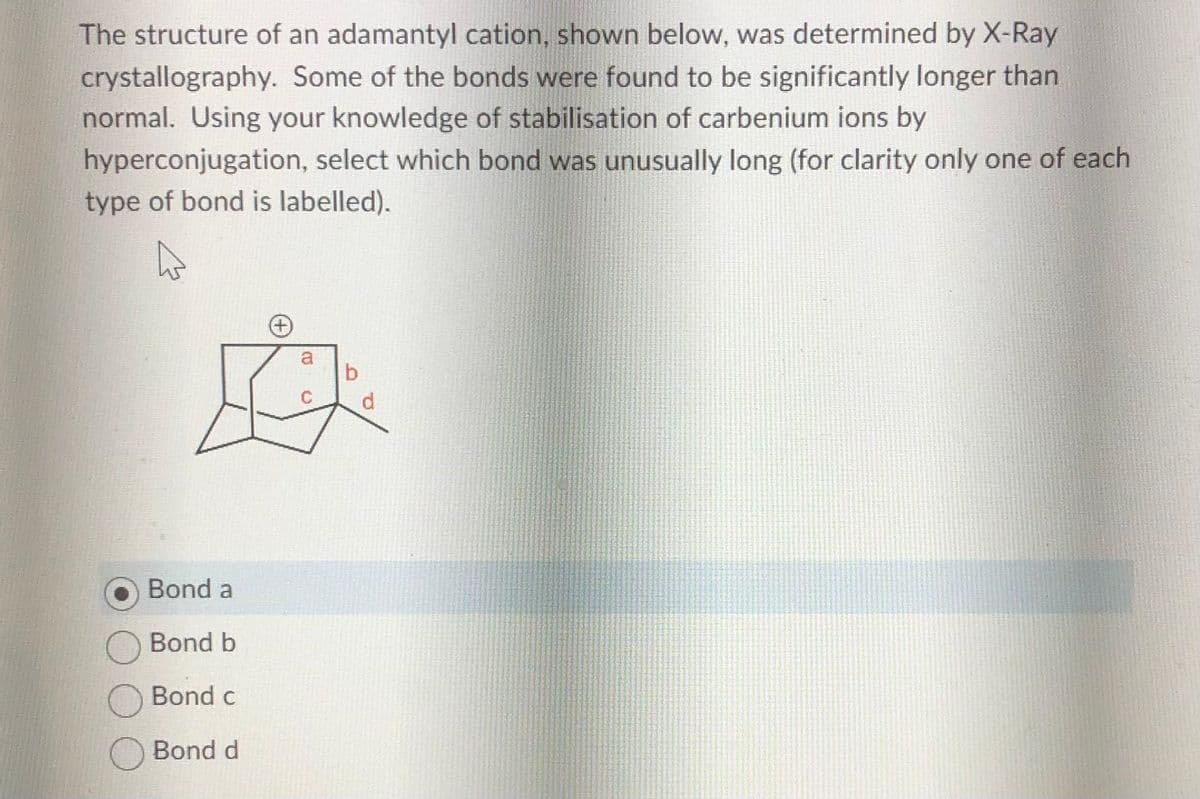
Transcribed Image Text:The structure of an adamantyl cation, shown below, was determined by X-Ray
crystallography. Some of the bonds were found to be significantly longer than
normal. Using your knowledge of stabilisation of carbenium ions by
hyperconjugation, select which bond was unusually long (for clarity only one of each
type of bond is labelled).
A
Bond a
Bond b
Bond c
O Bond d
+
B
b
d
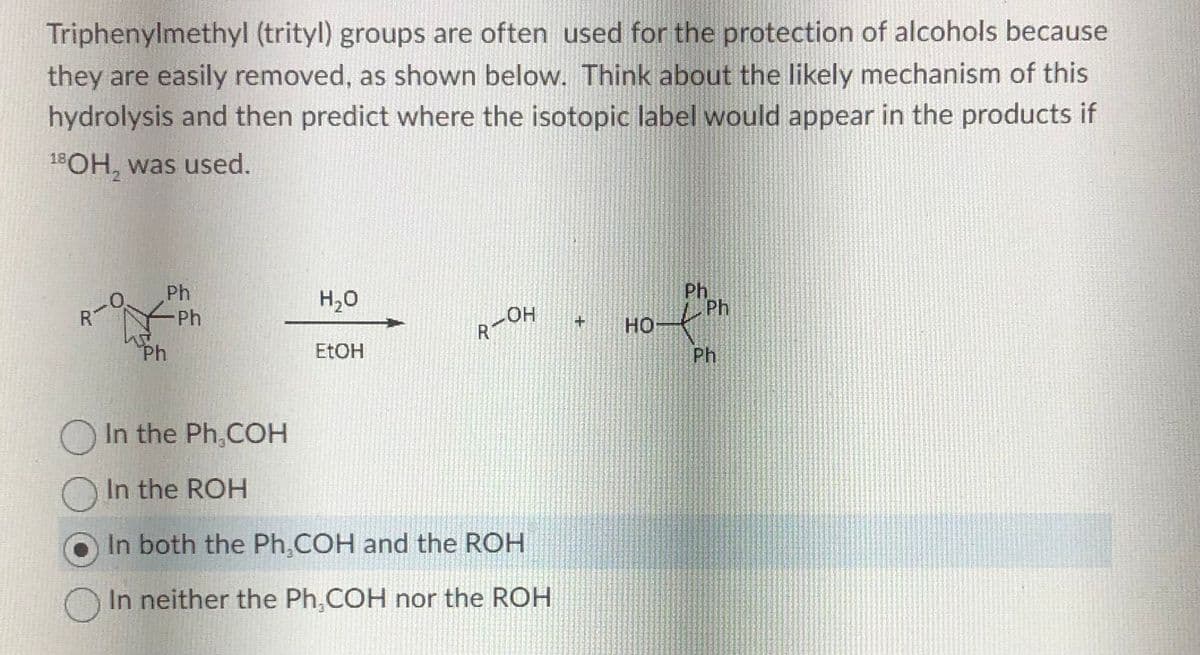
Transcribed Image Text:Triphenylmethyl (trityl) groups are often used for the protection of alcohols because
they are easily removed, as shown below. Think about the likely mechanism of this
hydrolysis and then predict where the isotopic label would appear in the products if
18OH, was used.
○
Ph
Ph
Ph
H₂O
EtOH
LOH
R-
In the Ph,COH
In the ROH
In both the Ph.COH and the ROH
In neither the Ph,COH nor the ROH
+
HO
Ph
Ph
Ph
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT
