TUTOR Percent Yield Consider this reaction, which occurs in the atmosphere and contributes to photochemical smog: H₂(g) + C₂H4(8) C₂H6(8) If there is 12.25 g H₂ and excess C₂H4 present, the reaction yields 150 g C₂H6. Calculate the percent yield for the reaction. Submit Show Approach Hide Tutor Steps TUTOR STEP Assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of the reactant. H₂. consumed by the reaction. Recheck 11th attempt Submit Answer Next (1 of 5) It is assumed that all of the reactant is converted to products, and none is lost. Try Another Version 10 item attempts remaining Approach initial mass of reactant Step 1 amount (mol) reactant. consumed Step 2 amount (mol) of product yielded Step 3 → mass of product yielded Step 1 Calculate amount (mol) of reactant converted to product from the mass, using the molar mass of the reactant. Step 2 Use the stoichiometric coefficients to determine the amount (mol) of product the reactant can produce.
TUTOR Percent Yield Consider this reaction, which occurs in the atmosphere and contributes to photochemical smog: H₂(g) + C₂H4(8) C₂H6(8) If there is 12.25 g H₂ and excess C₂H4 present, the reaction yields 150 g C₂H6. Calculate the percent yield for the reaction. Submit Show Approach Hide Tutor Steps TUTOR STEP Assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of the reactant. H₂. consumed by the reaction. Recheck 11th attempt Submit Answer Next (1 of 5) It is assumed that all of the reactant is converted to products, and none is lost. Try Another Version 10 item attempts remaining Approach initial mass of reactant Step 1 amount (mol) reactant. consumed Step 2 amount (mol) of product yielded Step 3 → mass of product yielded Step 1 Calculate amount (mol) of reactant converted to product from the mass, using the molar mass of the reactant. Step 2 Use the stoichiometric coefficients to determine the amount (mol) of product the reactant can produce.
Chemistry for Engineering Students
3rd Edition
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter4: Stoichiometry
Section: Chapter Questions
Problem 4.77PAE: The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce...
Related questions
Question
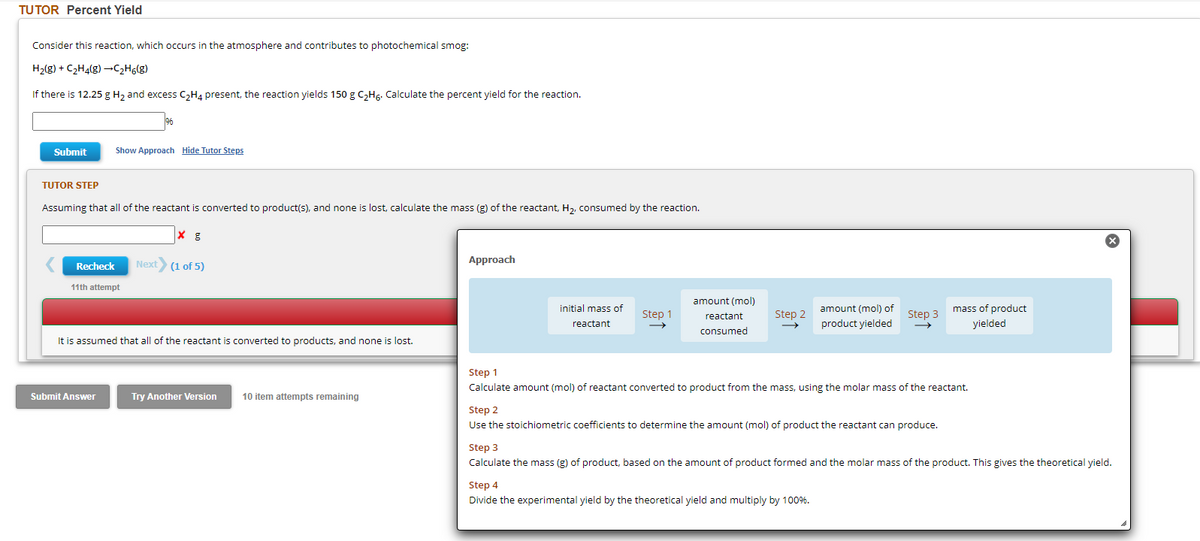
Transcribed Image Text:TUTOR Percent Yield
Consider this reaction, which occurs in the atmosphere and contributes to photochemical smog:
H₂(g) + C₂H4(g) →C₂H6(g)
If there is 12.25 g H₂ and excess C₂H4 present, the reaction yields 150 g C₂H6. Calculate the percent yield for the reaction.
Submit
TUTOR STEP
Assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of the reactant, H₂, consumed by the reaction.
Recheck
Show Approach Hide Tutor Steps
11th attempt
Submit Answer
Next (1 of 5)
It is assumed that all of the reactant is converted to products, and none i lost.
Try Another Version
10 item attempts remaining
Approach
initial mass of
reactant
Step 1
amount (mol)
reactant
consumed
Step 2
amount (mol) of
product yielded
Step 3
Step 1
Calculate amount (mol) of reactant converted to product from the mass, using the molar mass of the reactant.
Step 2
Use the stoichiometric coefficients to determine the amount (mol) of product the reactant can produce.
Step 4
Divide the experimental yield by the theoretical yield and multiply by 100%.
mass of product
yielded
Step 3
Calculate the mass (g) of product, based on the amount of product formed and the molar mass of the product. This gives the theoretical yield.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning