Two gases .I' and I are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to ultraviolet light the half-life of Xis 90. min, while that of Y' is 1.00 h. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L. flask with X and Y and exposes the flask to UV light. Initially, the partial pressure of X' is 75.0 % greater than the partial pressure of Y. As both gases decompose, will the partial pressure of X ever fall below the partial pressure of 1? Oyes 0.9 O no X ? If you said yes, calculate the time it takes the partial pressure of X to fall below the partial pressure of Y. Round your answer to 2 significant digits. min
Two gases .I' and I are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to ultraviolet light the half-life of Xis 90. min, while that of Y' is 1.00 h. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L. flask with X and Y and exposes the flask to UV light. Initially, the partial pressure of X' is 75.0 % greater than the partial pressure of Y. As both gases decompose, will the partial pressure of X ever fall below the partial pressure of 1? Oyes 0.9 O no X ? If you said yes, calculate the time it takes the partial pressure of X to fall below the partial pressure of Y. Round your answer to 2 significant digits. min
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 26QAP: When nitrogen dioxide reacts with carbon monoxide, the following reaction occurs. Â...
Related questions
Question
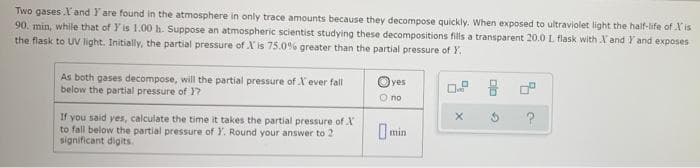
Transcribed Image Text:Two gases l'and I are found in the atmosphere in only trace amounts because they decompose quickly. When exposed to ultraviolet light the half-life of 'is
90. min, while that of Y' is 1.00 h. Suppose an atmospheric scientist studying these decompositions fills a transparent 20.0 L flask with X and Y and exposes
the flask to UV light. Initially, the partial pressure of X' is 75.0 % greater than the partial pressure of Y.
As both gases decompose, will the partial pressure of X' ever fall
below the partial pressure of Y?
Oyes
0.9
O no
?
If you said yes, calculate the time it takes the partial pressure of X
to fall below the partial pressure of Y. Round your answer to 2
significant digits.
min
X
G
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning