Two metals X and Y are mutually soluble in all proportions in the liquid state, but are completely insoluble in each other in the solid state. Metal X has melting temperature of 280° C while metal Y melts at 320°C. The metals forms a eutectic temperature 140°C and composition 60% X and 40%Y. a) Draw the binary X - Y phase diagram, and label all the phase fields and the phase transformation lines. b) From the phase diagram you have drawn describe the phase transformations that take place when the following two alloys are cooled from the liquid state to solid state under equilibrium conditions. i) An alloy containing 20% X and 80%Y. An alloys containing 80%X and 20%Y. ii)
Two metals X and Y are mutually soluble in all proportions in the liquid state, but are completely insoluble in each other in the solid state. Metal X has melting temperature of 280° C while metal Y melts at 320°C. The metals forms a eutectic temperature 140°C and composition 60% X and 40%Y. a) Draw the binary X - Y phase diagram, and label all the phase fields and the phase transformation lines. b) From the phase diagram you have drawn describe the phase transformations that take place when the following two alloys are cooled from the liquid state to solid state under equilibrium conditions. i) An alloy containing 20% X and 80%Y. An alloys containing 80%X and 20%Y. ii)
Chapter82: Physical Constants Of Liquids: The Boiling Point And Density
Section: Chapter Questions
Problem 5P
Related questions
Question
Need part a) and b) both in neat and clean handwriting.
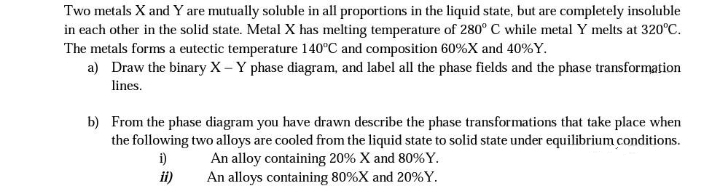
Transcribed Image Text:Two metals X and Y are mutually soluble in all proportions in the liquid state, but are completely insoluble
in each other in the solid state. Metal X has melting temperature of 280° C while metal Y melts at 320°C.
The metals forms a eutectic temperature 140°C and composition 60% X and 40%Y.
a) Draw the binary X - Y phase diagram, and label all the phase fields and the phase transformation
lines.
b) From the phase diagram you have drawn describe the phase transformations that take place when
the following two alloys are cooled from the liquid state to solid state under equilibrium conditions.
i)
An alloy containing 20% X and 80%Y.
An alloys containing 80%X and 20%Y.
ii)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT