7. A 0.4021-g sample of a purified organic acid was dissolved in water and titrated potentiometrically. A plot of the data revealed a single end point after 18.62 mL of 0.1243 M NaOH had been introduced. Calculate the molecular mass of the acid. ?
7. A 0.4021-g sample of a purified organic acid was dissolved in water and titrated potentiometrically. A plot of the data revealed a single end point after 18.62 mL of 0.1243 M NaOH had been introduced. Calculate the molecular mass of the acid. ?
Chapter21: Potentiometry
Section: Chapter Questions
Problem 21.22QAP
Related questions
Question
Please do this typewritten only. I will upvote. Show complete explanations or solutions. Thank you
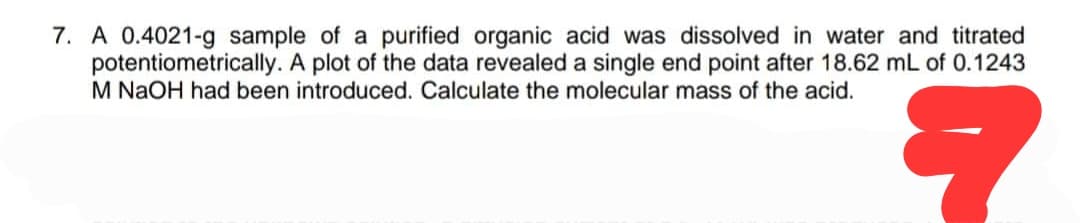
Transcribed Image Text:7. A 0.4021-g sample of a purified organic acid was dissolved in water and titrated
potentiometrically. A plot of the data revealed a single end point after 18.62 mL of 0.1243
M NaOH had been introduced. Calculate the molecular mass of the acid.
?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning