Under appropriate conditions A decomposes as follows: k=0.1/min A kz=0.1/min R R is to be produced from 1000 liter/hr of feed in which CAo = 1 mol/liter, CRO = Cso = 0. (a) What size of plug flow reactor will maximize the concentration of R, and what is that concentration in the effluent stream from this reactor? (b) What size of mixed flow reactor will maximize the concentration of R, and what is CR.max in the effluent stream from this reactor?
Under appropriate conditions A decomposes as follows: k=0.1/min A kz=0.1/min R R is to be produced from 1000 liter/hr of feed in which CAo = 1 mol/liter, CRO = Cso = 0. (a) What size of plug flow reactor will maximize the concentration of R, and what is that concentration in the effluent stream from this reactor? (b) What size of mixed flow reactor will maximize the concentration of R, and what is CR.max in the effluent stream from this reactor?
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.14QAP
Related questions
Question
i need the answer quickly
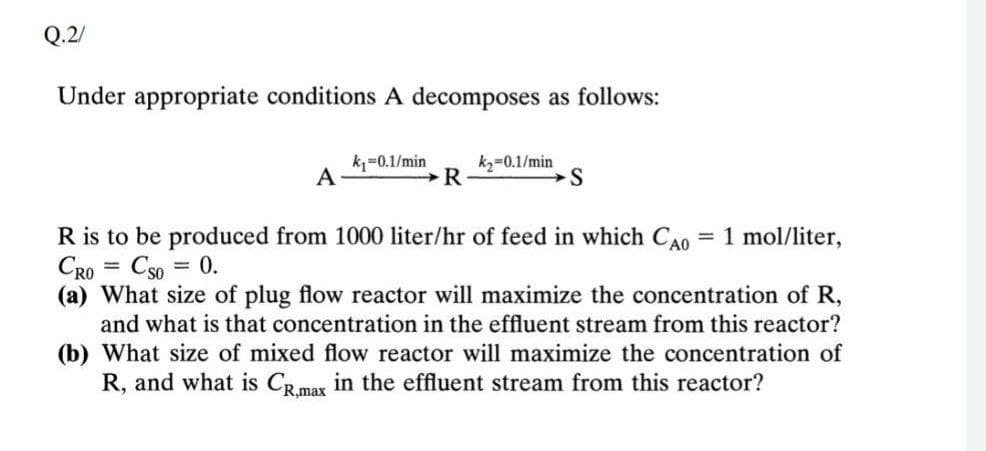
Transcribed Image Text:Q.2/
Under appropriate conditions A decomposes as follows:
k=0.1/min
A
kz=0.1/min
1 mol/liter,
R is to be produced from 1000 liter/hr of feed in which CAo
CRO
%3D
Cso = 0.
(a) What size of plug flow reactor will maximize the concentration of R,
and what is that concentration in the effluent stream from this reactor?
(b) What size of mixed flow reactor will maximize the concentration of
R, and what is CRmax in the effluent stream from this reactor?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

