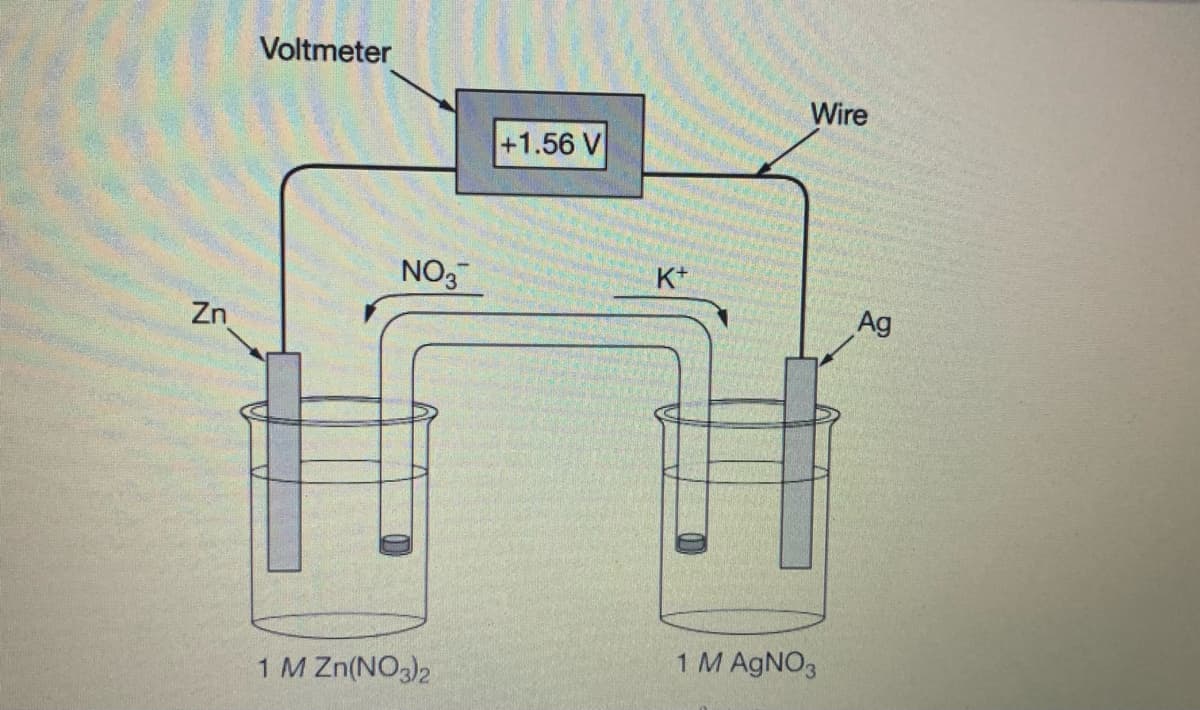
Under standard conditions, the galvanic cell shown above has a cell potential of+1.56 V using the reaction given. The salt bridge contains KNO3, which allows K+ ions and NO, ions to move in the directions indicated. If KNO3 in the salt bridge is replaced with KOH, some Zn(OH), (s) precipitates in the Zn-Zn(NO,), half-cell. Which of the following best explains how the cell potential is affected as Zn(OH), (s) starts to precipitate, and why? The cell potential increases because the concentration of Zn2+(ag) decreases and Q. ,becomes smaller. |Ag"* The cell potential decreases because the concentration of Zn2+ (aq) decreases and Q, Zn2 becomes smaller. The cell potential increases because Kt ions replace Zn?+ ions and the reduction of K* is more thermodynamically favored than the reduction of Zn?+. D The cell potential stays the same because Zn(OH), (s) is not part of the redox reaction responsible for the operation of the galvanic cel.
Under standard conditions, the galvanic cell shown above has a cell potential of+1.56 V using the reaction given. The salt bridge contains KNO3, which allows K+ ions and NO, ions to move in the directions indicated. If KNO3 in the salt bridge is replaced with KOH, some Zn(OH), (s) precipitates in the Zn-Zn(NO,), half-cell. Which of the following best explains how the cell potential is affected as Zn(OH), (s) starts to precipitate, and why? The cell potential increases because the concentration of Zn2+(ag) decreases and Q. ,becomes smaller. |Ag"* The cell potential decreases because the concentration of Zn2+ (aq) decreases and Q, Zn2 becomes smaller. The cell potential increases because Kt ions replace Zn?+ ions and the reduction of K* is more thermodynamically favored than the reduction of Zn?+. D The cell potential stays the same because Zn(OH), (s) is not part of the redox reaction responsible for the operation of the galvanic cel.
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.12QAP: What arc the advantages of microfabricated ISEs? Describe typical applications of this type of...
Related questions
Question

Transcribed Image Text:Under standard conditions, the galvanic cell shown above has a cell potential of+1.56 V using the reaction given. The salt bridge contains KNO3, which allows K ions and NO, ions to move in
the directions indicated. If KNO3 in the salt bridge is replaced with KOH, some Zn(OH), (s) precipitates in the Zn-Zn(NO,), half-cell. Which of the following best explains how the cell potential
is affected as Zn(OH), (s) starts to precipitate, and why?
The cell potential increases because the concentration of Zn2+(ag) decreases and Q.
,becomes smaller.
|Ag"
The cell potential decreases because the concentration of Zn2+ (ag) decreases and Q,
Zn
becomes smaller.
(Ag
The cell potential increases because Kt ions replace Zn2+ ions and the reduction of K* is more thermodynamically favored than the reduction of Zn?+.
D
The cell potential stays the same because Zn(OH), (8) is not part of the redox reaction responsible for the operation of the galvanic cell.
Transcribed Image Text:Voltmeter
Wire
+1.56 V
K+
NO3
Ag
Zn
1 M AgNO3
1 M Zn(NO3)2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning