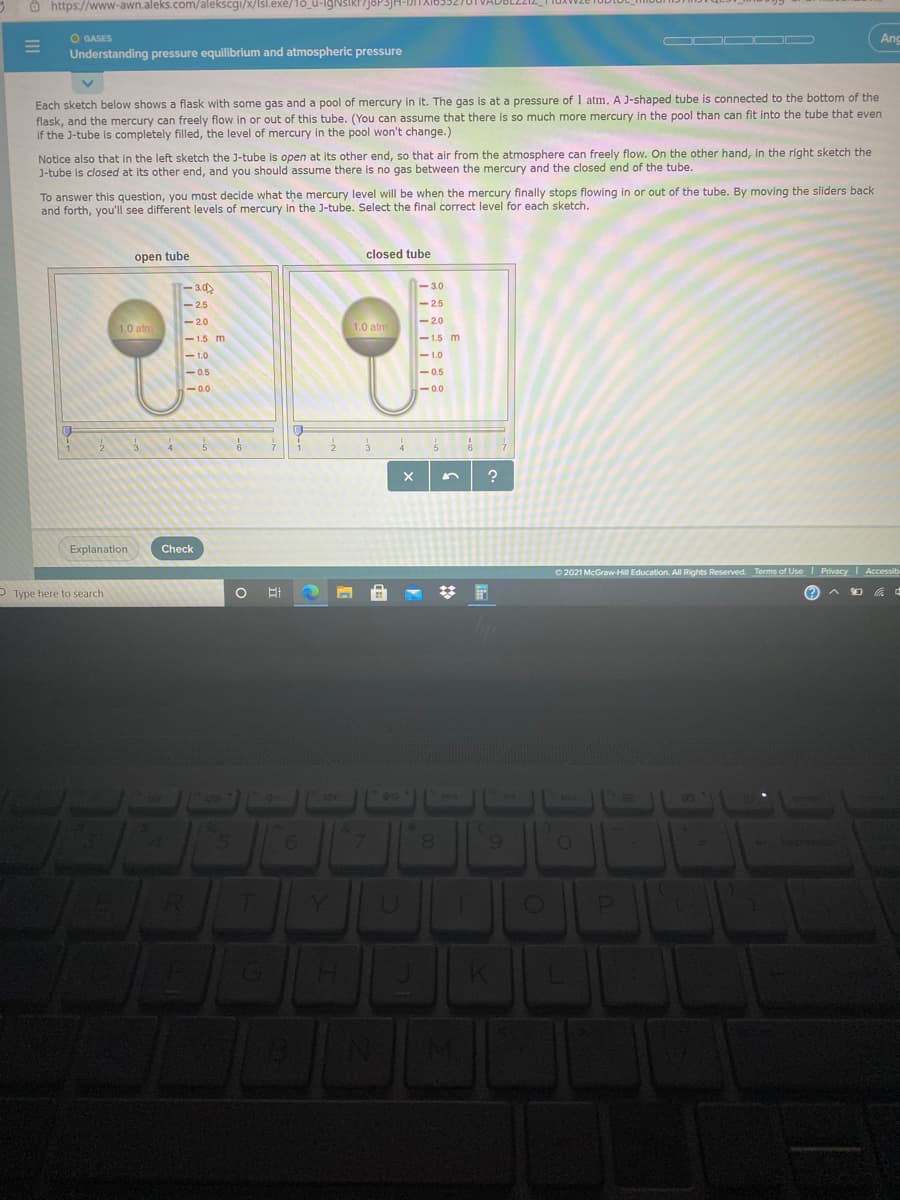
Understanding pressure equilibrium and atmospheric pressure Each sketch below shows a flask with some gas and a pool of mercury in it. The gas is at a pressure of 1 atm. A J-shaped tube is connected to the bottom of the flask, and the mercury can freely flow in or out of this tube. (You can assume that there is so much more mercury in the pool than can fit into the tube that even If the J-tube is completely filled, the level of mercury in the pool won't change.) Notice also that in the left sketch the J-tube is open at its other end, so that air from the atmosphere can freely flow. On the other hand, in the right sketch the J-tube is closed at its other end, and you should assume there is no gas between the mercury and the closed end of the tube. To answer this question, you mast decide what the mercury level will be when the mercury finally stops flowing in or out of the tube. By moving the sliders back and forth, you'll see different levels of mercury in the J-tube. Select the final correct level for each sketch. closed tube open tube - 3.0 -3.0 -2.5 -25 -2.0 -20 1.0 atm 1.0 atm - 1.5 m -1.5 m -1.0 -1.0 -0.5 -0.5 -0.0 -0.0
Understanding pressure equilibrium and atmospheric pressure Each sketch below shows a flask with some gas and a pool of mercury in it. The gas is at a pressure of 1 atm. A J-shaped tube is connected to the bottom of the flask, and the mercury can freely flow in or out of this tube. (You can assume that there is so much more mercury in the pool than can fit into the tube that even If the J-tube is completely filled, the level of mercury in the pool won't change.) Notice also that in the left sketch the J-tube is open at its other end, so that air from the atmosphere can freely flow. On the other hand, in the right sketch the J-tube is closed at its other end, and you should assume there is no gas between the mercury and the closed end of the tube. To answer this question, you mast decide what the mercury level will be when the mercury finally stops flowing in or out of the tube. By moving the sliders back and forth, you'll see different levels of mercury in the J-tube. Select the final correct level for each sketch. closed tube open tube - 3.0 -3.0 -2.5 -25 -2.0 -20 1.0 atm 1.0 atm - 1.5 m -1.5 m -1.0 -1.0 -0.5 -0.5 -0.0 -0.0
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter10: Entropy And The Second Law Of Thermodynamics
Section: Chapter Questions
Problem 10.67PAE
Related questions
Question
Answer all questions thank you ❤️
Transcribed Image Text:O https://www-awn.aleks.com/alekscgi/x/Isl.exé/18_u-lgNSIRI/J8P3JH-DITA163327
O GASES
Understanding pressure equilibrium and atmospheric pressure
Ang
Each sketch below shows a flask with some gas and a pool of mercury in it. The gas is at a pressure of 1 atm. A J-shaped tube is connected to the bottom of the
flask, and the mercury can freely flow in or out of this tube. (You can assume that there is so much more mercury in the pool than can fit into the tube that even
if the J-tube is completely filled, the level of mercury in the pool won't change.)
Notice also that in the left sketch the J-tube is open at its other end, so that air from the atmosphere can freely flow. On the other hand, in the right sketch the
J-tube is closed at its other end, and you should assume there is no gas between the mercury and the closed end of the tube.
To answer this question, you must decide what the mercury level will be when the mercury finally stops flowing in or out of the tube. By moving the sliders back
and forth, you'll see different levels of mercury in the J-tube. Select the final correct level for each sketch.
open tube
closed tube
T-3.0
-3.0
-2.5
-2.5
-20
-20
1.0 atm
1.0 atm
-1.5 m
-1,5 m
- 1.0
-1.0
-0.5
-0.5
- 0.5
-0.0
-0.0
6.
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use I Privacy Accessib
P Type here to search
ロ d
4+
90
6.
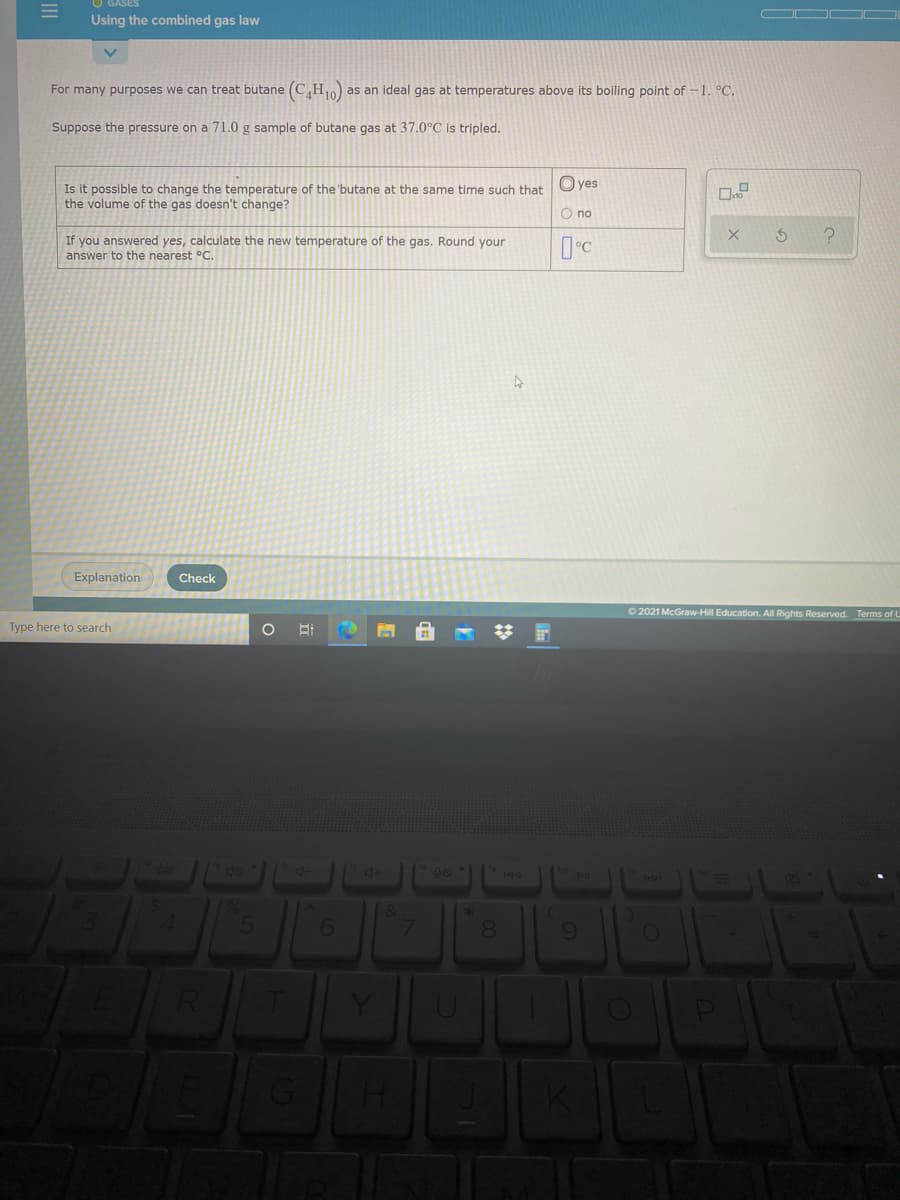
Transcribed Image Text:Using the combined gas law
For many purposes we can treat butane (C,H as an ideal gas at temperatures above its boiling point of -1. °C.
Suppose the pressure on a 71.0 g sample of butane gas at 37.0°C is tripled.
O yes
Is it possible to change the temperature of the 'butane at the same time such that
the volume of the gas doesn't change?
O no
If you answered yes, calculate the new temperature of the gas. Round your
answer to the nearest °C.
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of L
Type here to search
144
6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning