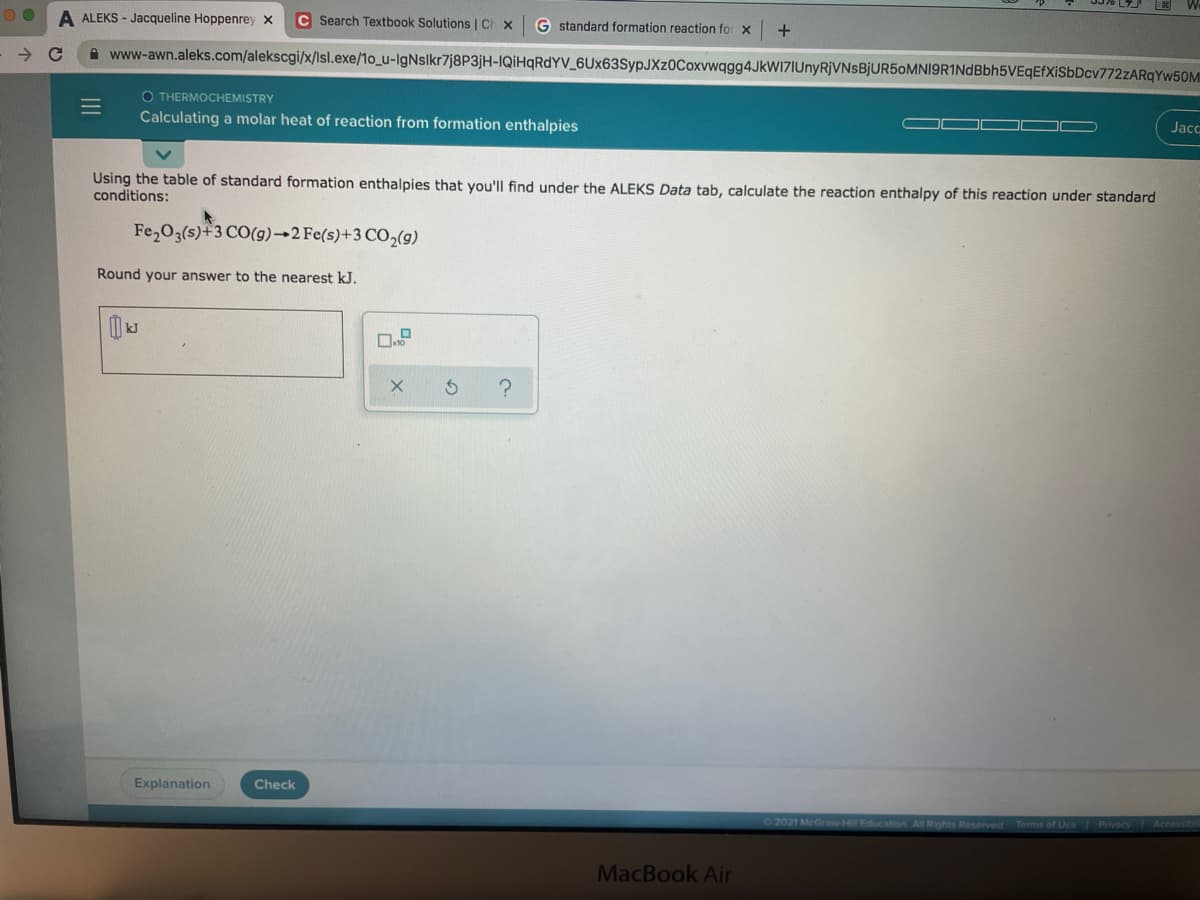
uon enthalples Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: Fe,O3(s)+3 CO(g)→2 Fe(s)+3 CO,(g) Round your answer to the nearest kJ.
uon enthalples Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard conditions: Fe,O3(s)+3 CO(g)→2 Fe(s)+3 CO,(g) Round your answer to the nearest kJ.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 3RQ: Table 17-1 lists common half-reactions along with the standard reduction potential associated with...
Related questions
Question
100%
Transcribed Image Text:A ALEKS - Jacqueline Hoppenrey x
C Search Textbook Solutions | Ch x
G standard formation reaction for x
A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWIZIUnyRjVNsBjUR5oMNI9R1NdBbh5VEqEfXiSbDcv772zARqYw50M
O THERMOCHEMISTRY
Calculating a molar heat of reaction from formation enthalpies
Jacc
Using the table of standard formation enthalpies that you'll find under the ALEKS Data tab, calculate the reaction enthalpy of this reaction under standard
conditions:
Fe,O3(s)+3 CO(g)→2 Fe(s)+3 CO,(g)
Round your answer to the nearest kJ.
Explanation
Check
2021 McGraw.Hill Education All Rights Reserved Temms of Uss Privacy Accessic
MacBook Air
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning