Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction: Ca2*(aq) + 2Cu*(aq)Cd(s) + 2Cu²*(aq) Answer: kJ K for this reaction would be than one. Cu2*(aq) + 2 e → Cu(s) 0.337 Cu2*(aq) + e Cu"(aq) 0.153 –→ S(s) + 2 H"(aq) + 2 e¯ →H2S(aq) 0.14 +. 2 H"(aq) + 2 e →H2(g) 0.0000 Pb2+(aq) + 2 e Pb(s) -0.126 Sn2+ (aq) + 2 e. → Sn(s) -0.14 Ni²+, (aq) + 2 e . Ni(s) -0.25 Co2+(aq) + 2 e → Co(s) -0.28 Cd2* (aq) + 2 e¯ → Cd(s) -0.403
Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction: Ca2*(aq) + 2Cu*(aq)Cd(s) + 2Cu²*(aq) Answer: kJ K for this reaction would be than one. Cu2*(aq) + 2 e → Cu(s) 0.337 Cu2*(aq) + e Cu"(aq) 0.153 –→ S(s) + 2 H"(aq) + 2 e¯ →H2S(aq) 0.14 +. 2 H"(aq) + 2 e →H2(g) 0.0000 Pb2+(aq) + 2 e Pb(s) -0.126 Sn2+ (aq) + 2 e. → Sn(s) -0.14 Ni²+, (aq) + 2 e . Ni(s) -0.25 Co2+(aq) + 2 e → Co(s) -0.28 Cd2* (aq) + 2 e¯ → Cd(s) -0.403
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter13: Electrochemistry
Section: Chapter Questions
Problem 13.21PAE: Using values from the table of standard reduction potentials, calculate the cell potentials of the...
Related questions
Question
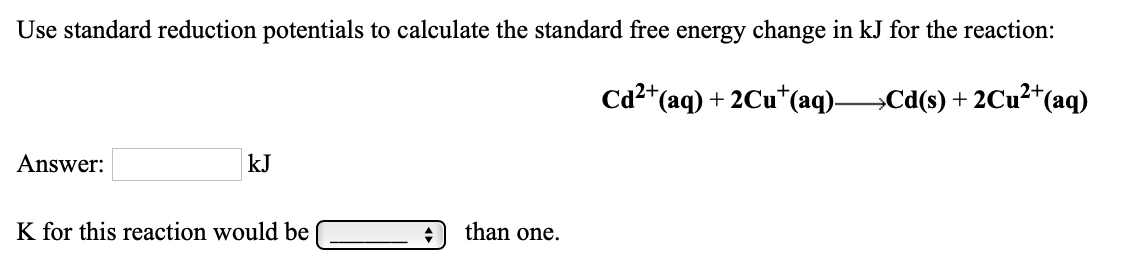
Transcribed Image Text:Use standard reduction potentials to calculate the standard free energy change in kJ for the reaction:
Ca2*(aq) + 2Cu*(aq)Cd(s) + 2Cu²*(aq)
Answer:
kJ
K for this reaction would be
than one.

Transcribed Image Text:Cu2*(aq) + 2 e → Cu(s)
0.337
Cu2*(aq) + e
Cu"(aq)
0.153
–→
S(s) + 2 H"(aq) + 2 e¯ →H2S(aq)
0.14
+.
2 H"(aq) + 2 e →H2(g)
0.0000
Pb2+(aq) + 2 e
Pb(s)
-0.126
Sn2+
(aq) + 2 e.
→ Sn(s)
-0.14
Ni²+,
(aq) + 2 e .
Ni(s)
-0.25
Co2+(aq) + 2 e
→ Co(s)
-0.28
Cd2* (aq) + 2 e¯ → Cd(s)
-0.403
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co