Use the bond energy to estimate the heat of reaction at 25 °C for the reaction below. CH4 (g) + 2 02 (g) → CO2 (g) + 2 H2O(g) Compare your result with that calculated from enthalpies of formation.
Use the bond energy to estimate the heat of reaction at 25 °C for the reaction below. CH4 (g) + 2 02 (g) → CO2 (g) + 2 H2O(g) Compare your result with that calculated from enthalpies of formation.
Chemistry: Matter and Change
1st Edition
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Chapter15: Energy And Chemical Change
Section15.4: Calculating Enthalpy Change
Problem 35PP
Related questions
Question
100%

Transcribed Image Text:Use the bond energy to estimate the heat of reaction at 25 °C for the reaction below.
CH4 (g) + 2 02 (g) → CO2 (g) + 2 H2O(g)
Compare your result with that calculated from enthalpies of formation.
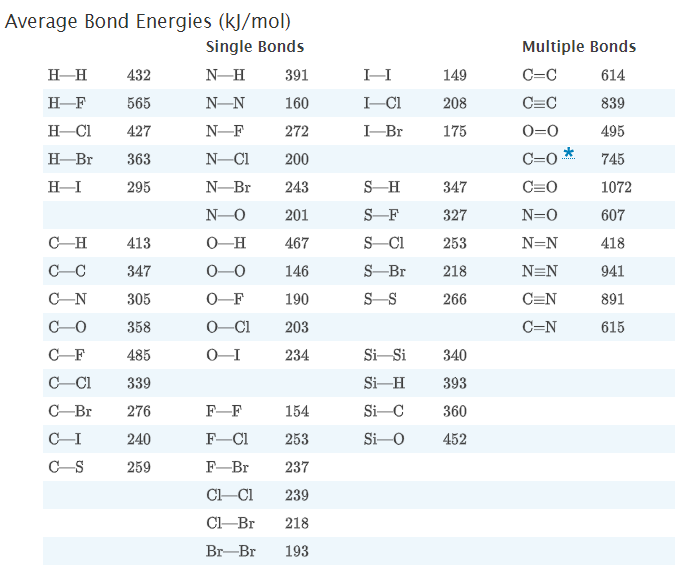
Transcribed Image Text:Average Bond Energies (kJ/mol)
Single Bonds
Multiple Bonds
H-H
432
NH
391
149
C=C
614
H-F
565
N-N
160
208
C=C
839
H-CI
427
N-F
272
-Br
175
O=0
495
H-Br
363
N-CI
200
C=0
745
HI
295
N-Br
243
S H
347
C=0
1072
N-O
201
SF
327
N=0
607
C-H
413
O-H
467
S CI
253
N=N
418
C-C
347
0-0
146
S Br
218
N=N
941
C-N
305
O-F
190
266
C=N
891
C-O
358
O-C
203
C=N
615
C-F
485
234
Si Si
340
C-Cl
339
Si-H
393
C-Br
276
F-F
154
Si-C
360
240
F-Cl
253
Si-O
452
C-S
259
F-Br
237
C-CI
239
ClBr
218
Br-Br
193
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning