The chemistry of nitrogen oxides is very versatile. Given the following reactions and their standard enthalpy changes, Ан° (1) NOg)NO2(g) N203(g) =-39.8 kJ + rxn Ан° (2) NO(g) NO2(g) + O2(g) N205(g) =-112.5 kJ rxn Ан° N204(8) (3) 2NO2(g) -57.2 kJ rxn Дн" %3-114.2 kJ (4) 2NO(g) 02g) 2NO2(g) = rxn Ан° subl (5) N205(s) N205(g) = 54.1 kJ Calculate the heat of reaction for AH N203(g)N205(s) 2N204(g) kJ
The chemistry of nitrogen oxides is very versatile. Given the following reactions and their standard enthalpy changes, Ан° (1) NOg)NO2(g) N203(g) =-39.8 kJ + rxn Ан° (2) NO(g) NO2(g) + O2(g) N205(g) =-112.5 kJ rxn Ан° N204(8) (3) 2NO2(g) -57.2 kJ rxn Дн" %3-114.2 kJ (4) 2NO(g) 02g) 2NO2(g) = rxn Ан° subl (5) N205(s) N205(g) = 54.1 kJ Calculate the heat of reaction for AH N203(g)N205(s) 2N204(g) kJ
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter5: Thermochemistry
Section: Chapter Questions
Problem 5.86QE: One of the components of jet engine fuel is n-dodecane, C12H26(), which has a standard enthalpy of...
Related questions
Question
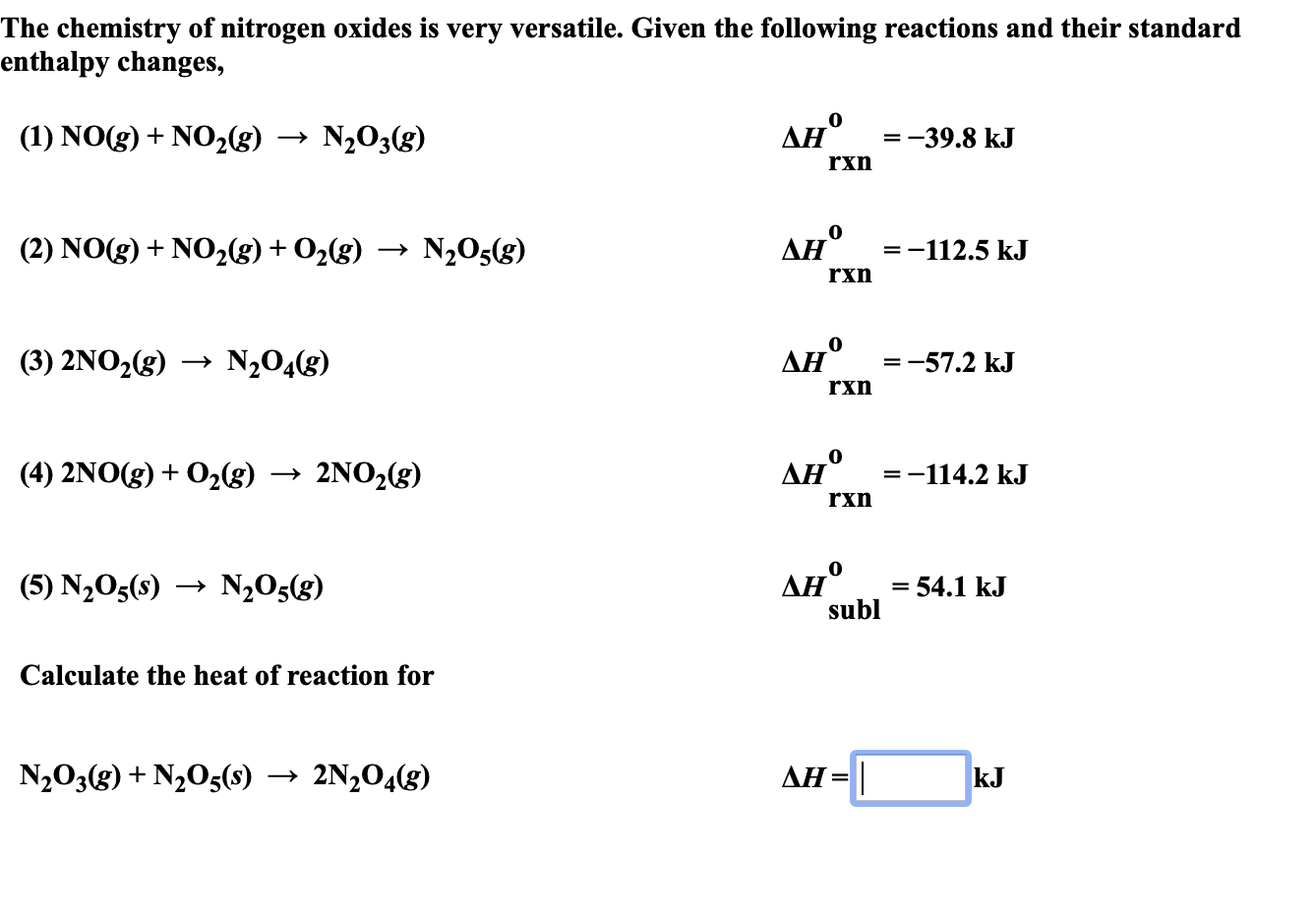
Transcribed Image Text:The chemistry of nitrogen oxides is very versatile. Given the following reactions and their standard
enthalpy changes,
Ан°
(1) NOg)NO2(g)
N203(g)
=-39.8 kJ
+
rxn
Ан°
(2) NO(g) NO2(g) + O2(g)
N205(g)
=-112.5 kJ
rxn
Ан°
N204(8)
(3) 2NO2(g)
-57.2 kJ
rxn
Дн" %3-114.2 kJ
(4) 2NO(g) 02g)
2NO2(g)
=
rxn
Ан°
subl
(5) N205(s)
N205(g)
= 54.1 kJ
Calculate the heat of reaction for
AH
N203(g)N205(s)
2N204(g)
kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
