Use the figure to answer this question. Order these nuclei based upon the strength of their nuclear binding energies from weakes to strongest. 10 slope b (Fe 235 Fusion Fission H. 50 150 200 250 100 Number of nucleons Drag and drop options into correct order and submit. For keyboard navigation. SHOW MORE V 160 4He 12C slope a 4) 2. Binding energy per nucleons (MeV) II II
Use the figure to answer this question. Order these nuclei based upon the strength of their nuclear binding energies from weakes to strongest. 10 slope b (Fe 235 Fusion Fission H. 50 150 200 250 100 Number of nucleons Drag and drop options into correct order and submit. For keyboard navigation. SHOW MORE V 160 4He 12C slope a 4) 2. Binding energy per nucleons (MeV) II II
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter18: Nuclear Chemistry
Section: Chapter Questions
Problem 23QRT
Related questions
Question
3
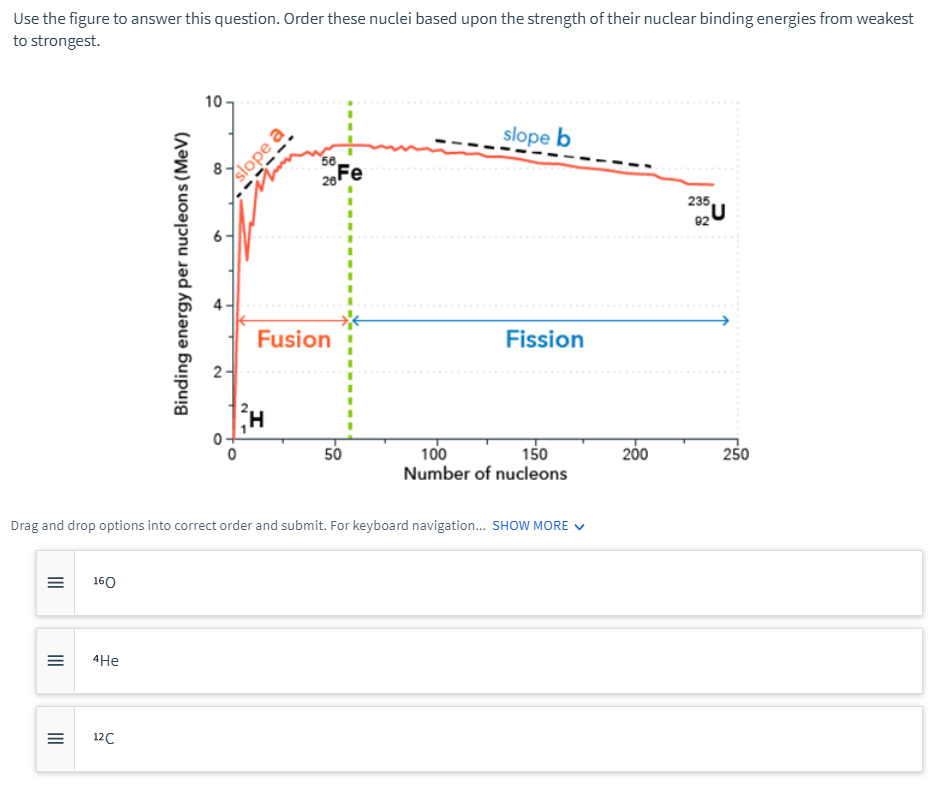
Transcribed Image Text:Use the figure to answer this question. Order these nuclei based upon the strength of their nuclear binding energies from weakest
to strongest.
10
slope b
56,
235
92
Fusion
Fission
2-
100
Number of nucleons
50
150
200
250
Drag and drop options into correct order and submit. For keyboard navigation. SHOW MORE V
160
4He
12C
slope a
Binding energy per nucleons (MeV)
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax