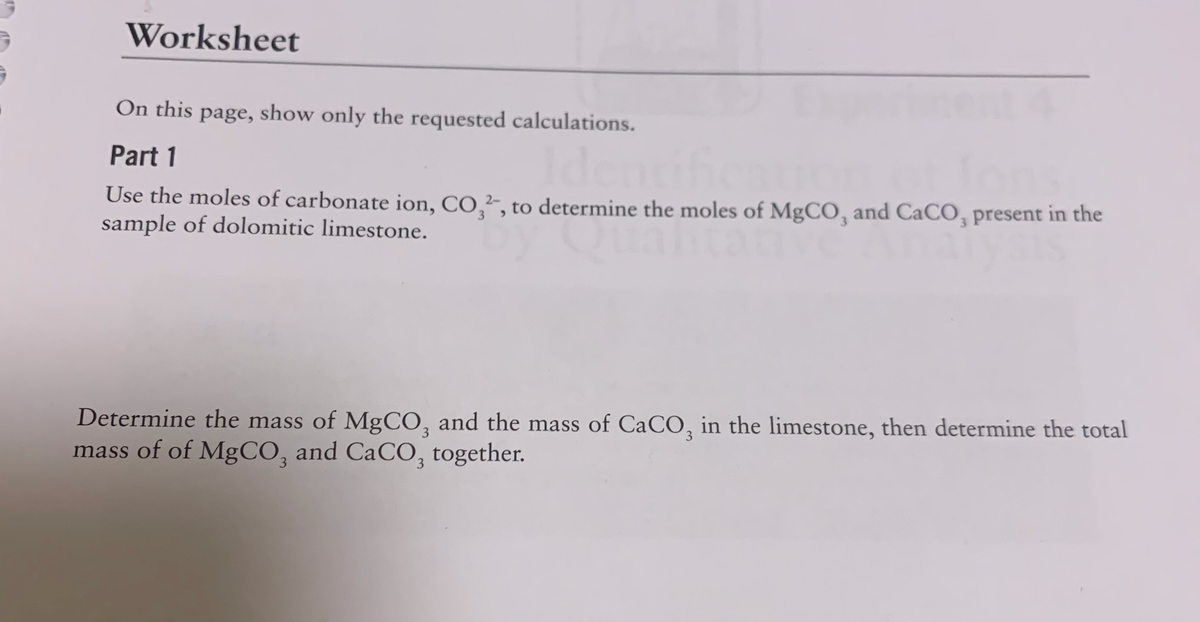
Use the moles of carbonate ion, CO,²-, to determine the moles of MgCO, and CaCO, present in the sample of dolomitic limestone. Determine the mass of MgCO, and the mass of CaCO, in the limestone, then determine the total mass of of MgCO, and CaCO, together.
Use the moles of carbonate ion, CO,²-, to determine the moles of MgCO, and CaCO, present in the sample of dolomitic limestone. Determine the mass of MgCO, and the mass of CaCO, in the limestone, then determine the total mass of of MgCO, and CaCO, together.
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter3: Composition Of Substances And Solutions
Section: Chapter Questions
Problem 77E: Copper(I) iodide (CuI) is often added to table salt as a dietary source of iodine. How many moles of...
Related questions
Question
I need help with these questions using my calculated values!!!
Transcribed Image Text:Worksheet
On this page, show only the requested calculations.
Part 1
Use the moles of carbonate ion, CO,², to determine the moles of MgCO, and CaCO, present in the
sample of dolomitic limestone.
2-
3.
Determine the mass of MgCO, and the mass of CaCO, in the limestone, then determine the total
3.
3
mass of of MgCO, and CaCO, together.

Transcribed Image Text:Calculated Values
Limestone
Oyster Shell
0.7975g
१५.१५০
Mass of sample
98.0435g
0.3366g
Initial mass of flask + acid + sample
0-3201g
0-0072734 mol
Mass of CO, lost at 20 min
Moles of CO,
నయరిరె
Moles of CO,-
0.0072734 mol
6.0076483 md
0,0036367 mol
00076483 mol
Moles of CaCO,
Moles of MgCO,
0,0036367 md
Total mass of MGCO, + CACO,
G.670161
34.089028
Mass percent (MgCO, + CaCO,)
6,765494
Mass of CaCO,
99.454332
Mass percent CaCO,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER