Use the observation in the first column to answer the question in the second column. observation At 85 °C, Substance E has a vapor pressure of 108. torr and Substance F has a vapor pressure of 98. torr. The enthalpy of vaporization of Substance C is smaller than that of Substance D. At 1 atm pressure, Substance A boils at 135. °C and Substance B boils at 165. °C. question Which has a higher boiling point? Substance E O Substance F O Neither, E and F have the same boiling point. O It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? O Substance C O Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher enthalpy of vaporization? O Substance A O Substance Neither, A and B have the same enthalpy of vaporization. O It's impossible to know without more information.
Use the observation in the first column to answer the question in the second column. observation At 85 °C, Substance E has a vapor pressure of 108. torr and Substance F has a vapor pressure of 98. torr. The enthalpy of vaporization of Substance C is smaller than that of Substance D. At 1 atm pressure, Substance A boils at 135. °C and Substance B boils at 165. °C. question Which has a higher boiling point? Substance E O Substance F O Neither, E and F have the same boiling point. O It's impossible to know without more information. At any temperature where both substances are liquid, which has the higher vapor pressure? O Substance C O Substance D Neither, C and D have the same vapor pressure. It's impossible to know without more information. Which has a higher enthalpy of vaporization? O Substance A O Substance Neither, A and B have the same enthalpy of vaporization. O It's impossible to know without more information.
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter8: Molecules And Materials
Section: Chapter Questions
Problem 8.87PAE: 8.87 Use the vapor pressure curves illustrated here to answer the questions that follow. (a) What is...
Related questions
Question
Solve correctly please.
Need all subparts solution with explanation also.
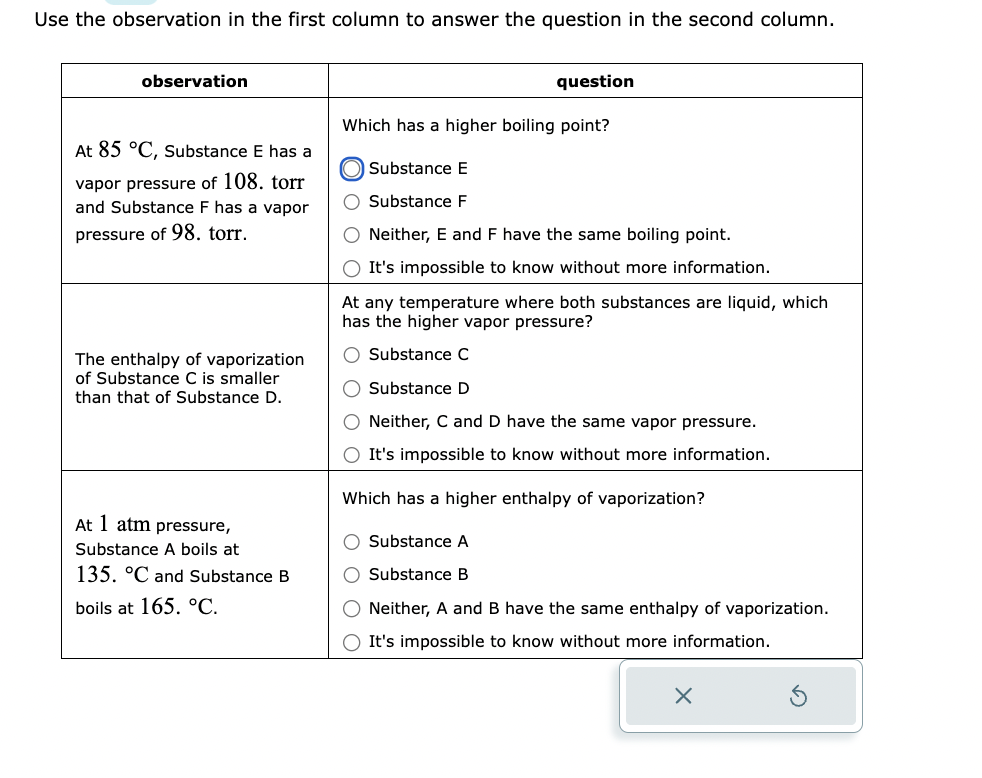
Transcribed Image Text:Use the observation in the first column to answer the question in the second column.
observation
At 85 °C, Substance E has a
vapor pressure of 108. torr
and Substance F has a vapor
pressure of 98. torr.
The enthalpy of vaporization
of Substance C is smaller
than that of Substance D.
At 1 atm pressure,
Substance A boils at
135. °C and Substance B
boils at 165. °C.
question
Which has a higher boiling point?
Substance E
O Substance F
O Neither, E and F have the same boiling point.
O It's impossible to know without more information.
At any temperature where both substances are liquid, which
has the higher vapor pressure?
O Substance C
Substance D
O Neither, C and D have the same vapor pressure.
O It's impossible to know without more information.
Which has a higher enthalpy of vaporization?
O Substance A
O Substance B
Neither, A and B have the same enthalpy of vaporization.
O It's impossible to know without more information.
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
