Use the References to access important values if needed for th 1. What is the mass of 1.53x1024 atoms of helium? grams 2. How many helium atoms are there in 46.4 grams of helium? atoms Submit Answer 5 question attempts remaining
Use the References to access important values if needed for th 1. What is the mass of 1.53x1024 atoms of helium? grams 2. How many helium atoms are there in 46.4 grams of helium? atoms Submit Answer 5 question attempts remaining
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter2: Atoms And Molecules
Section: Chapter Questions
Problem 2.24E: Round atomic weights to the nearest whole number, and determine how many helium atoms would balance...
Related questions
Question
Transcribed Image Text:Use the References to access important values if needed for this q
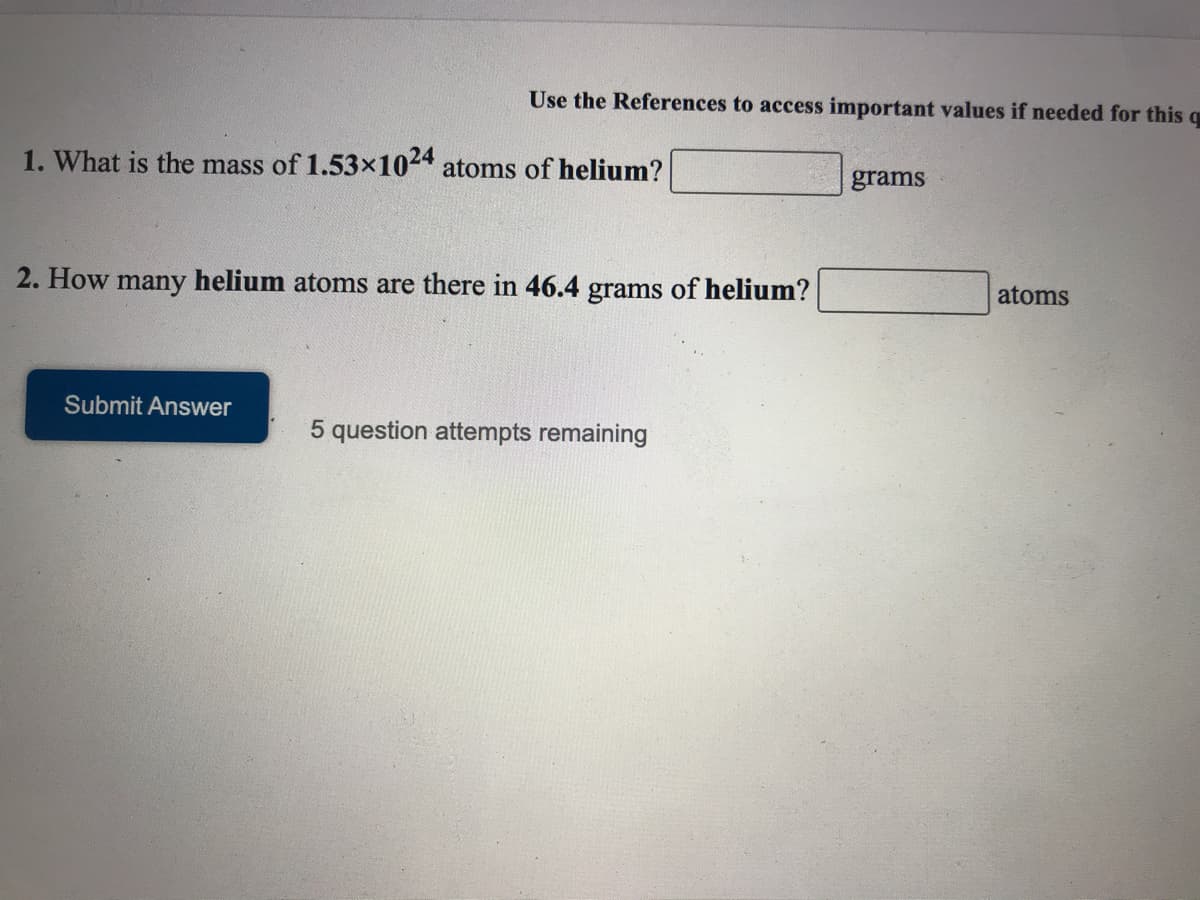
1. What is the mass of 1.53x1024 atoms of helium?
grams
2. How many helium atoms are there in 46.4 grams of helium?
atoms
Submit Answer
5 question attempts remaining
Expert Solution
Step 1
We know that:
1 mole of a substance consist of 6.022*1023 atoms.
(A) We need to calculate the mass of 1.53*1024 atoms of Helium:
We know that: 1 mole of He atom weighs 4.0026 g.
ie 4.0026 g of He consist of 6.022*1023 atoms.
Thus, 1.53*1024 atoms of He will weigh (4.0026 g/6.022*1023 atoms)*(1.53*1024 atoms)
= 10.17 grams
Thus, 1.53*1024 atoms of He will weigh 10.17 grams.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning