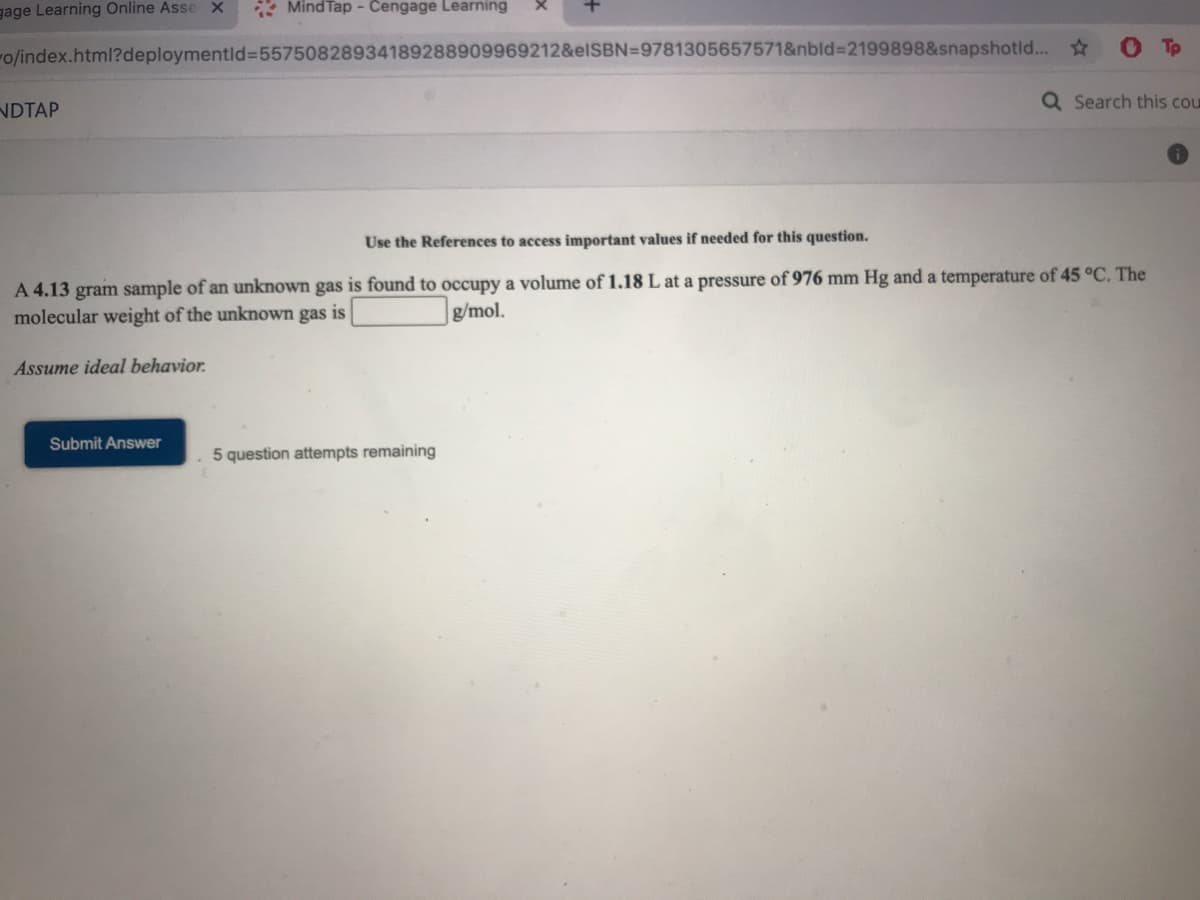
Use the References to access important values if needed for this question. A 4.13 gram sample of an unknown gas is found to occupy a volume of 1.18 L at a pressure of 976 mm Hg and a temperature of 45 °C. The molecular weight of the unknown gas is g/mol. Assume ideal behavior. Submit Answer 5 question attempts remaining
Use the References to access important values if needed for this question. A 4.13 gram sample of an unknown gas is found to occupy a volume of 1.18 L at a pressure of 976 mm Hg and a temperature of 45 °C. The molecular weight of the unknown gas is g/mol. Assume ideal behavior. Submit Answer 5 question attempts remaining
Chapter6: Random Errors In Chemical Analysis
Section: Chapter Questions
Problem 6.22QAP
Related questions
Question
Transcribed Image Text:gage Learning Online Asse x
* Mind Tap - Čengage Learning
ro/index.html?deploymentld%355750828934189288909969212&elSBN=9781305657571&nbld%3D2199898&snapshotld... ☆
NDTAP
Q Search this cou
Use the References to access important values if needed for this question.
A 4.13 gram sample of an unknown gas is found to occupy a volume of 1.18 L at a pressure of 976 mm Hg and a temperature of 45 °C. The
molecular weight of the unknown gas is
g/mol.
Assume ideal behavior.
Submit Answer
5 question attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

