Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Question
For the equation 2K + Cl2------>2KCl. I understand Cl is diatomic , but where does the 2 in front of the K come from.
Transcribed Image Text:10:04 M
Answered: upplemental Problems.
https://www.bartleby.com/questions-and-ans..
= bartleby
A Q&A I 0
Science / Chemis... / Q&A Libr... / upplemental Problems f...
upplemental Problems from Chapter 2 CC...
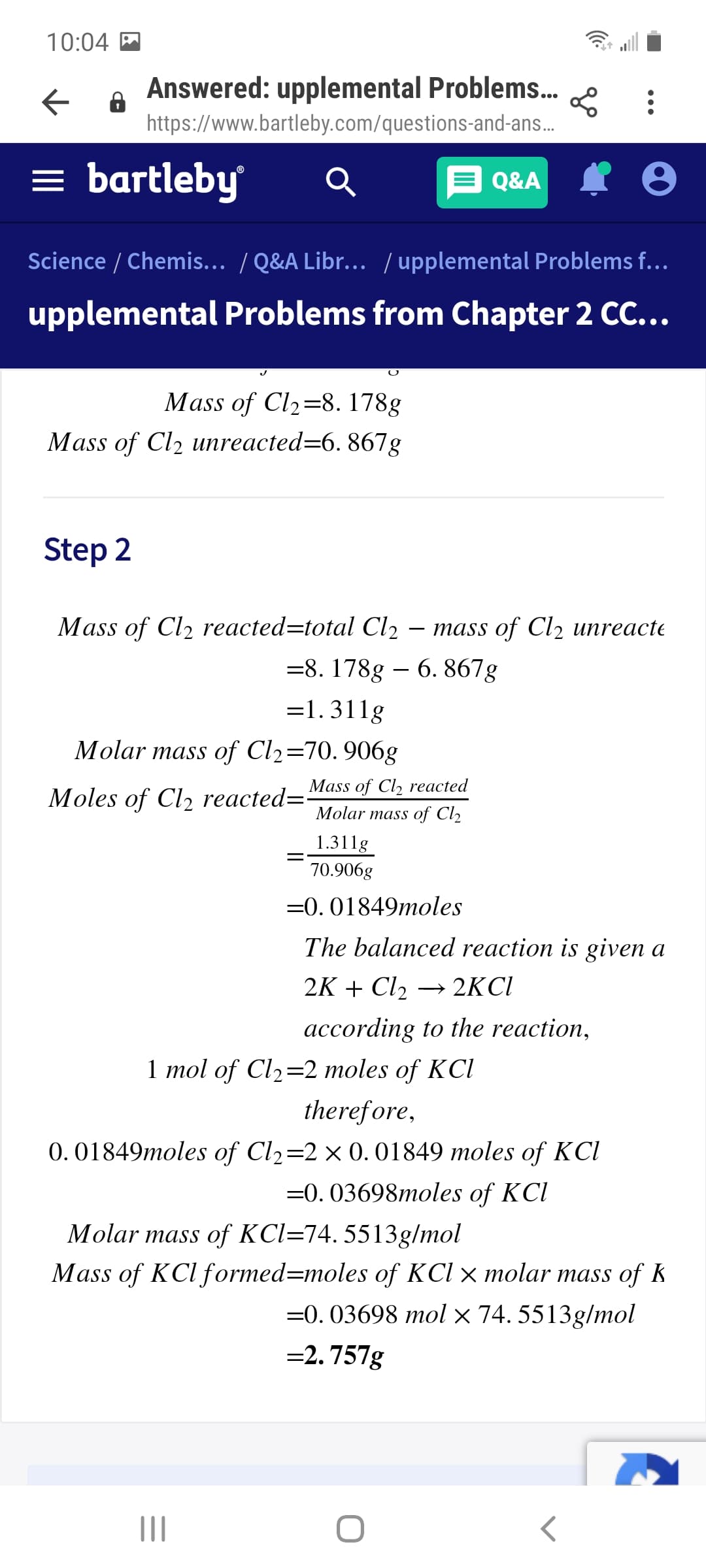
Mass of Cl2=8. 178g
Mass of Cl2 unreacted=6. 867g
Step 2
Mass of Cl2 reacted=total Cl2
тass of Clz иnreacte
=8. 178g – 6. 867g
=1.311g
Molar mass of Cl2=70. 906g
Mass of Cl, reacted
Moles of Cl2 reacted="
Molar mass of Cl2
1.311g
70.906g
=0. 01849moles
The balanced reaction is given a
2K + Cl2
→ 2KCI
according to the reaction,
1 mol of Cl2=2 moles of KCl
therefore,
0. 01849moles of Cl2=2× 0.01849 moles of KCI
=0. 03698moles of KCl
Molar mass of KCl=74. 5513g/mol
Mass of KCl formed=moles of KCl × molar mass of k
=0. 03698 mol × 74. 5513g/mol
=2. 757g
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



