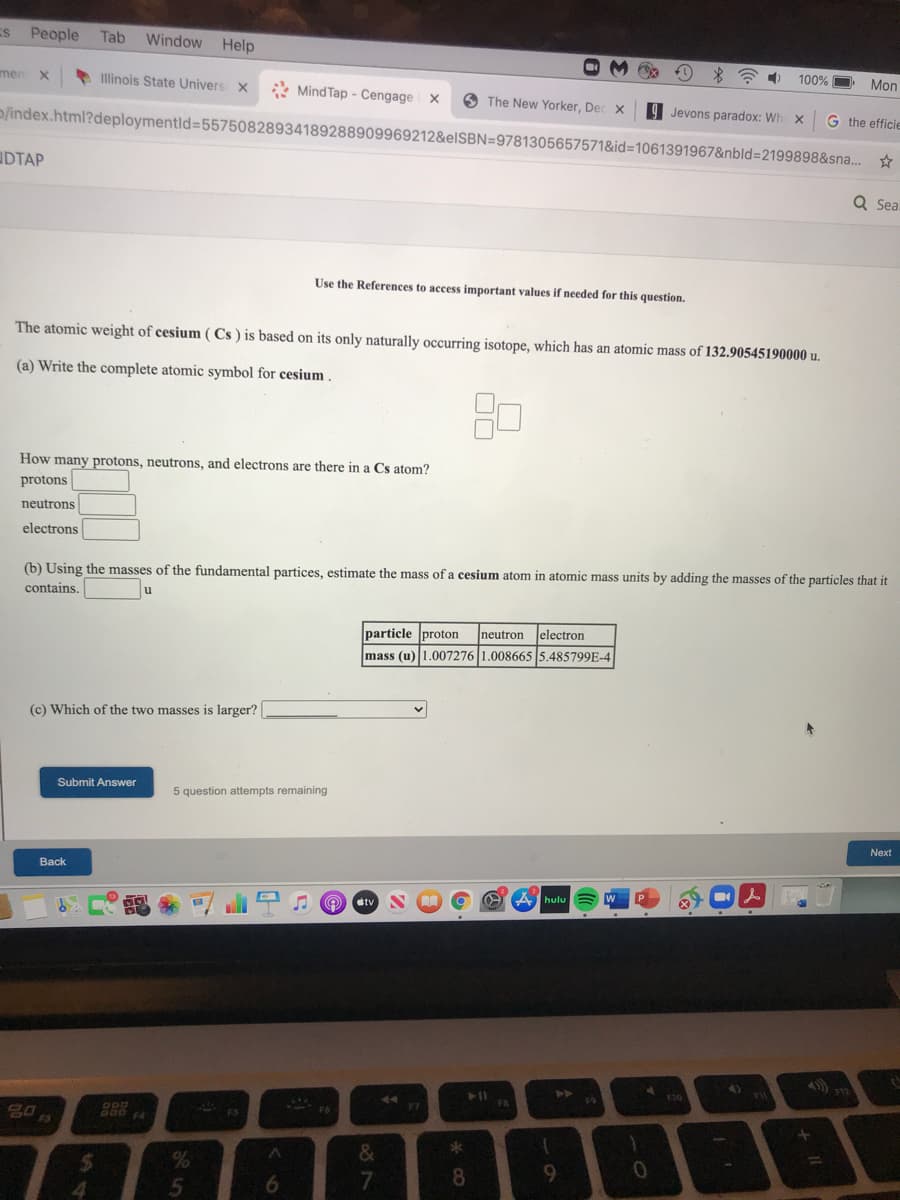
Use the References to access important values if needed for this question. The atomic weight of cesium ( Cs ) is based on its only naturally occurring isotope, which has an atomic mass of 132.90545190000 u. (a) Write the complete atomic symbol for cesium. How many protons, neutrons, and electrons are there in a Cs atom? protons neutrons electrons (b) Using the masses of the fundamental partices, estimate the mass of a cesium atom in atomic mass units by adding the masses of the particles that in contains. particle proton neutron electron mass (u) 1.007276 1.008665 5.485799E-4 (c) Which of the two masses is larger? Submit Answer 5 question attempts remaining
Use the References to access important values if needed for this question. The atomic weight of cesium ( Cs ) is based on its only naturally occurring isotope, which has an atomic mass of 132.90545190000 u. (a) Write the complete atomic symbol for cesium. How many protons, neutrons, and electrons are there in a Cs atom? protons neutrons electrons (b) Using the masses of the fundamental partices, estimate the mass of a cesium atom in atomic mass units by adding the masses of the particles that in contains. particle proton neutron electron mass (u) 1.007276 1.008665 5.485799E-4 (c) Which of the two masses is larger? Submit Answer 5 question attempts remaining
Chapter14: Chromatography
Section: Chapter Questions
Problem 9P
Related questions
Concept explainers
Atomic Structure
The basic structure of an atom is defined as the component-level of atomic structure of an atom. Precisely speaking an atom consists of three major subatomic particles which are protons, neutrons, and electrons. Many theories have been stated for explaining the structure of an atom.
Shape of the D Orbital
Shapes of orbitals are an approximate representation of boundaries in space for finding electrons occupied in that respective orbital. D orbitals are known to have a clover leaf shape or dumbbell inside where electrons can be found.
Question
Transcribed Image Text:People
Tab
Window Help
100% O
Mon
men x
> Illinois State Univers x
* Mind Tap - Cengage x
O The New Yorker, Dec x
9 Jevons paradox: Wh x
G the efficie
p/index.html?deploymentld%=55750828934189288909969212&elSBN=9781305657571&id=1061391967&nbld=2199898&sna..
DTAP
Q Sea
Use the References to access important values if needed for this question.
The atomic weight of cesium ( Cs ) is based on its only naturally occurring isotope, which has an atomic mass of 132.90545190000 u.
(a) Write the complete atomic symbol for cesium
How many
cons, neutrons, and electrons are there in a Cs atom?
protons
neutrons
electrons
(b) Using the masses of the fundamental partices, estimate the mass of a cesium atom in atomic mass units by adding the masses of the particles that it
contains
u
particle proton
Ineutron lelectron
mass (u)1.007276 1.008665 5.485799E-4
(c) Which of the two masses is larger?
Submit Answer
5 question attempts remaining
Next
Back
hulu
etv
4)
FI
FB
64
20
13
F4
&
%24
7.
4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

