Use the simulation lab to obtain the distance for the solvent front and the distance for each spot and then calculate the Rf 35ig figs acetaminoph Unknown Mixture #1 Unknown Sample caffeine ibuprofen aspirin en Mixture #2 13.0 cm13.0 cm 13. O cm 13.0 cm 13.0cm Distance to 130 cm solvent front 1.77cm 1.89cm 1.78cm la .6 cm 11.0 cm 6.27cm Distance 11.1 Spot(s) traveled 5. 58cm 12.6 cm Calculated R:(s) Components of Unknowns D. Additional Exercises 1. Which of the substances tested is most polar? What parts of the structure of this substance is polar? 2. Which of the substance tested is most non-polar? What parts of the structure of this substance in non-polar?
Use the simulation lab to obtain the distance for the solvent front and the distance for each spot and then calculate the Rf 35ig figs acetaminoph Unknown Mixture #1 Unknown Sample caffeine ibuprofen aspirin en Mixture #2 13.0 cm13.0 cm 13. O cm 13.0 cm 13.0cm Distance to 130 cm solvent front 1.77cm 1.89cm 1.78cm la .6 cm 11.0 cm 6.27cm Distance 11.1 Spot(s) traveled 5. 58cm 12.6 cm Calculated R:(s) Components of Unknowns D. Additional Exercises 1. Which of the substances tested is most polar? What parts of the structure of this substance is polar? 2. Which of the substance tested is most non-polar? What parts of the structure of this substance in non-polar?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter27: Gas Chromatography
Section: Chapter Questions
Problem 27.24QAP
Related questions
Question
2
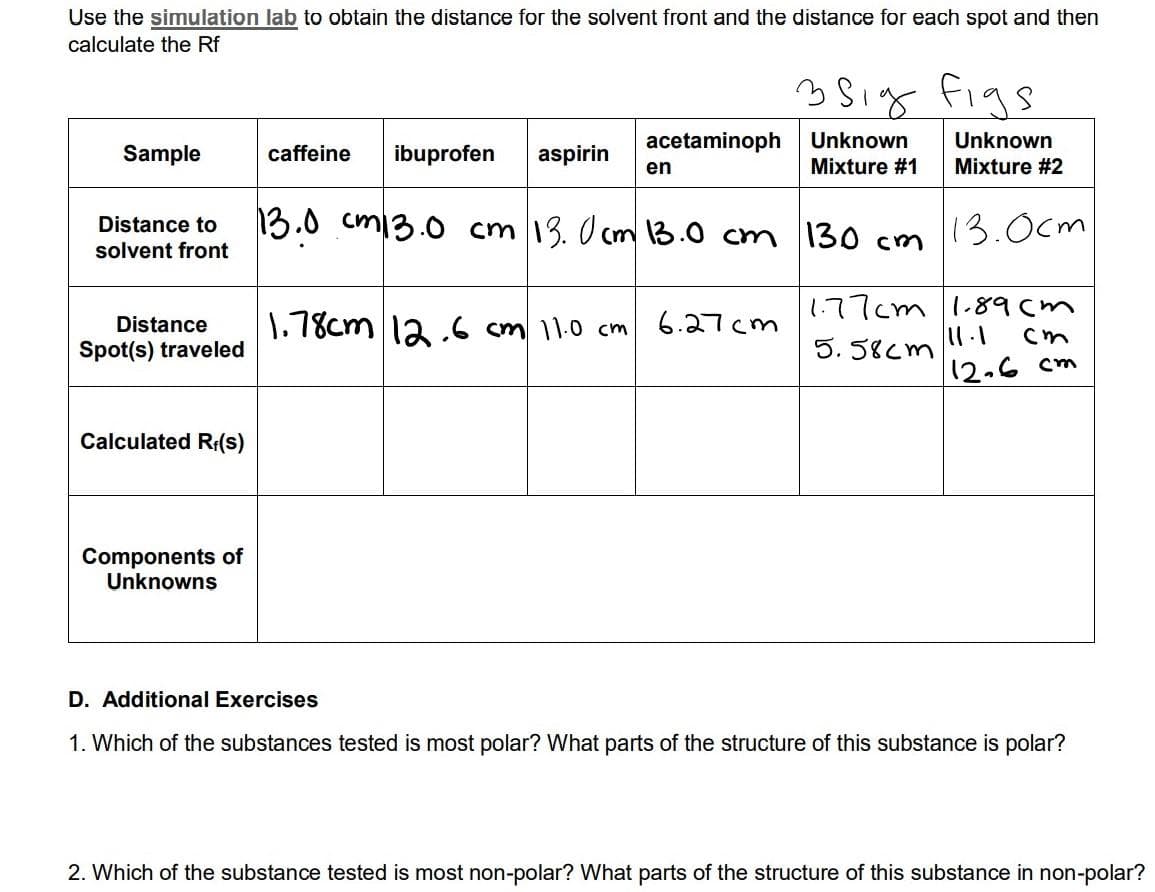
Transcribed Image Text:Use the simulation lab to obtain the distance for the solvent front and the distance for each spot and then
calculate the Rf
35ig figs
acetaminoph
Unknown
Unknown
Sample
caffeine
ibuprofen
aspirin
en
Mixture #1
Mixture #2
13.0 cm13.0 cm 13. O cm 13.0 cm
13.0cm
Distance to
130 cm
solvent front
1.77cm 1.89cm
1.78cm la.6 cm 11.0 cm
6.27cm
Distance
11.1
Spot(s) traveled
5. 58cm
12.6 cm
Calculated R:(s)
Components of
Unknowns
D. Additional Exercises
1. Which of the substances tested is most polar? What parts of the structure of this substance is polar?
2. Which of the substance tested is most non-polar? What parts of the structure of this substance in non-polar?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co