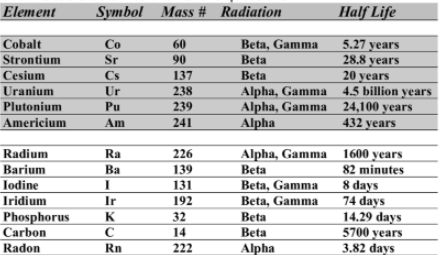
Use the table below to answer the questions. In your own words, define half-life. b. Which isotope on the table is the MOST stable? c. Which isotope on the table is the LEAST stable? d. Explain how you determined your answers to b and c.
Use the table below to answer the questions. In your own words, define half-life. b. Which isotope on the table is the MOST stable? c. Which isotope on the table is the LEAST stable? d. Explain how you determined your answers to b and c.
Chapter19: The Nucleus: A Chemist's View
Section: Chapter Questions
Problem 6Q
Related questions
Question
Use the table below to answer the questions.
In your own words, define half-life.
b. Which isotope on the table is the MOST stable?
c. Which isotope on the table is the LEAST stable?
d. Explain how you determined your answers to b and c.
e. Write the balanced decay reactions for 60Co, 238U, 131I, and 222Rn.
Transcribed Image Text:Element
Symbol Mass # Radiation
Half Life
Beta, Gamma
Cobalt
Strontium
Cesium
Co
60
5.27 years
28,8 years
Sr
90
Beta
20 years
Alpha, Gamma 4.5 billion years
Alpha, Gamma 24,100 years
432 years
Cs
137
Beta
Uranium
Ur
238
Plutonium
Pu
239
Americium
Am
241
Alpha
Alpha, Gamma 1600 years
82 minutes
8 days
74 days
14.29 days
5700 years
Radium
Ra
226
Barium
Ва
139
Beta
Beta, Gamma
Beta, Gamma
Iodine
Iridium
Phosphorus
Carbon
131
Ir
192
K
32
Beta
Beta
14
222
Radon
Rn
Alpha
3.82 days
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax