Use the table of standard bond energies given below to calculate the energy of reaction for the following chemical reaction. H O C= O H-H + O C O H Standard Single Bond Energies (in kJ/mol) H C O F CI H 432 411 346 459 358 142 565 155 485 190 428 327 218 249 240 Standard Multiple Bond Energies C=C C=0 0=0 CEC 602 799 494 835 Answer Enter your answer here Back Leave blank Next COF C
Use the table of standard bond energies given below to calculate the energy of reaction for the following chemical reaction. H O C= O H-H + O C O H Standard Single Bond Energies (in kJ/mol) H C O F CI H 432 411 346 459 358 142 565 155 485 190 428 327 218 249 240 Standard Multiple Bond Energies C=C C=0 0=0 CEC 602 799 494 835 Answer Enter your answer here Back Leave blank Next COF C
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter9: Chemical Bonds
Section: Chapter Questions
Problem 9.21QE
Related questions
Question
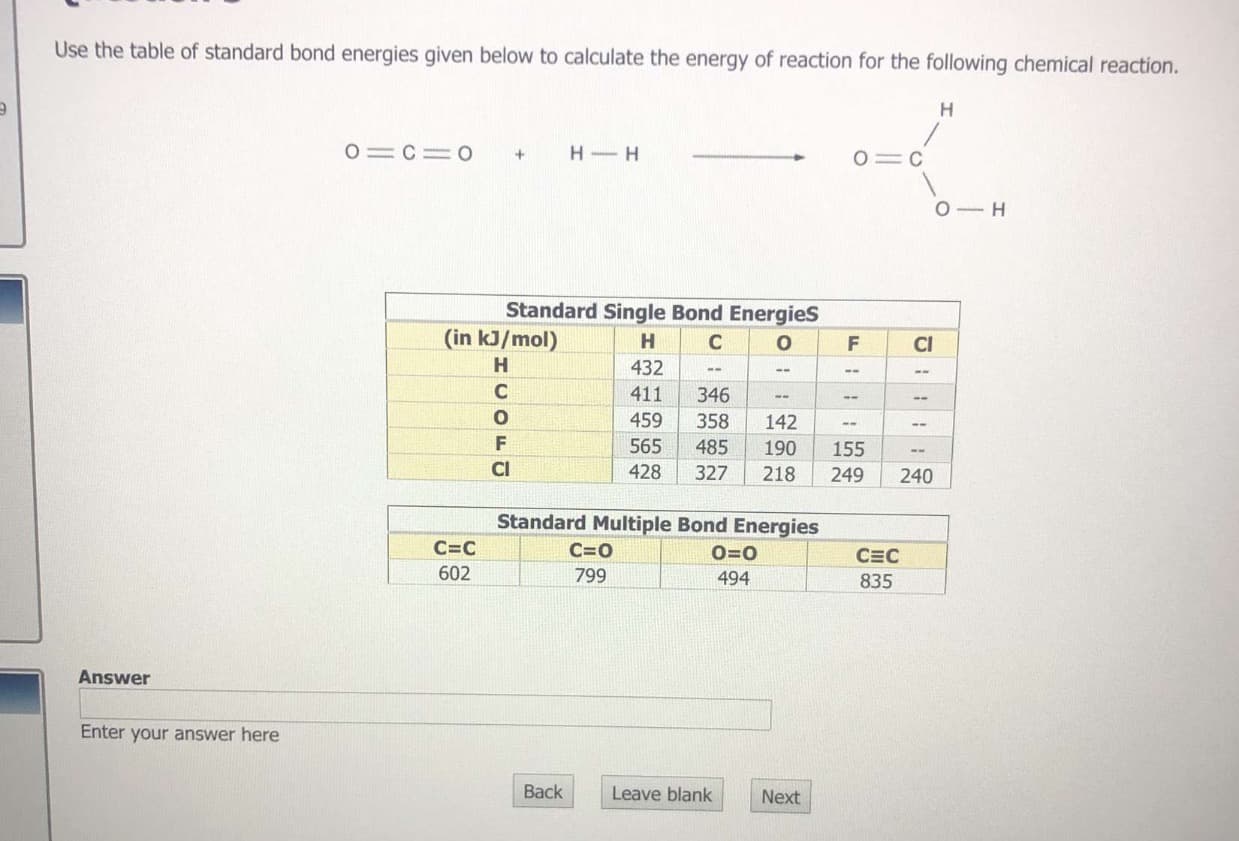
Transcribed Image Text:Use the table of standard bond energies given below to calculate the energy of reaction for the following chemical reaction.
H
O C= O
H-H
+
O C
O H
Standard Single Bond Energies
(in kJ/mol)
H
C
O
F
CI
H
432
411
346
459
358
142
565
155
485
190
428
327
218
249
240
Standard Multiple Bond Energies
C=C
C=0
0=0
CEC
602
799
494
835
Answer
Enter your answer here
Back
Leave blank
Next
COF C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning