Given the data below, H°, for the reaction rxn 2SO2 (g) + O2 (g) → 2SO3 (g) is kJ. AH°; (SO2 (g)) = -297 kJ/mol AH°; (SO3 (g)) = -396 kJ/mol AH°; (SO2C12 (g)) = -364 kJ/mol AH°f (H2SO2 (1)) = -814 kJ/mol AH°; (G2O (1)) = -286 kJ/mol %3D O The AH°F of O2 (g) is needed for the calculation. 99 198 -198 -99
Given the data below, H°, for the reaction rxn 2SO2 (g) + O2 (g) → 2SO3 (g) is kJ. AH°; (SO2 (g)) = -297 kJ/mol AH°; (SO3 (g)) = -396 kJ/mol AH°; (SO2C12 (g)) = -364 kJ/mol AH°f (H2SO2 (1)) = -814 kJ/mol AH°; (G2O (1)) = -286 kJ/mol %3D O The AH°F of O2 (g) is needed for the calculation. 99 198 -198 -99
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter5: Principles Of Chemical Reactivity: Energy And Chemical Reactions
Section5.5: Enthalpy Changes For Chemical Reactions
Problem 1RC: 1. For the reaction 2 Hg(l) + O2(g) → 2 HgO(s), ∆rH° = 181.6 kJ/mol-rxn. What is the enthalpy change...
Related questions
Question
100%
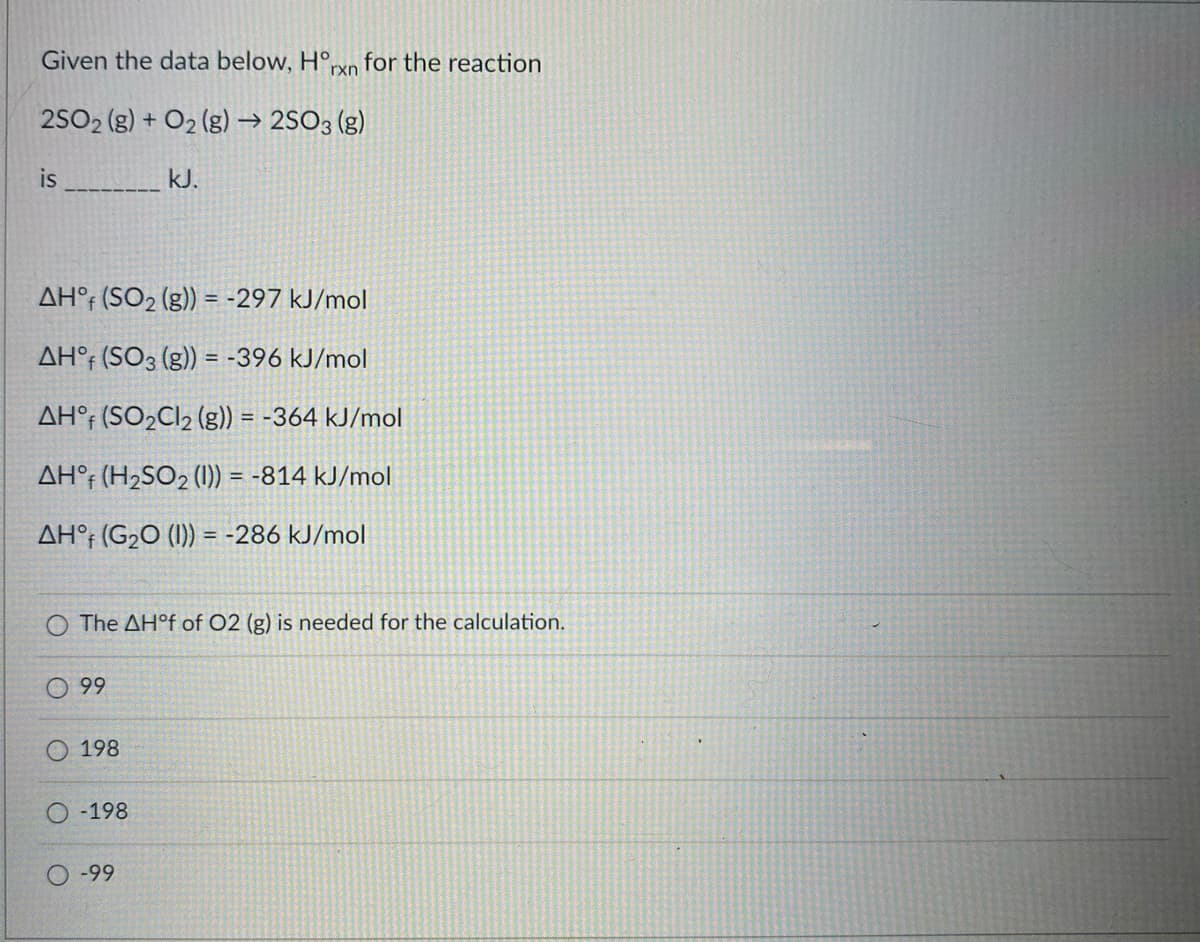
Transcribed Image Text:Given the data below, H°,
for the reaction
rxn
2SO2 (g) + O2 (g) → 2SO3 (g)
is
kJ.
AH°; (SO2 (g)) = -297 kJ/mol
AH°; (SO3 (g)) = -396 kJ/mol
AH°; (SO2C12 (g)) = -364 kJ/mol
AH°f (H2SO2 (1)) = -814 kJ/mol
AH°; (G2O (1)) = -286 kJ/mol
%3D
O The AH°F of O2 (g) is needed for the calculation.
99
198
-198
-99
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
