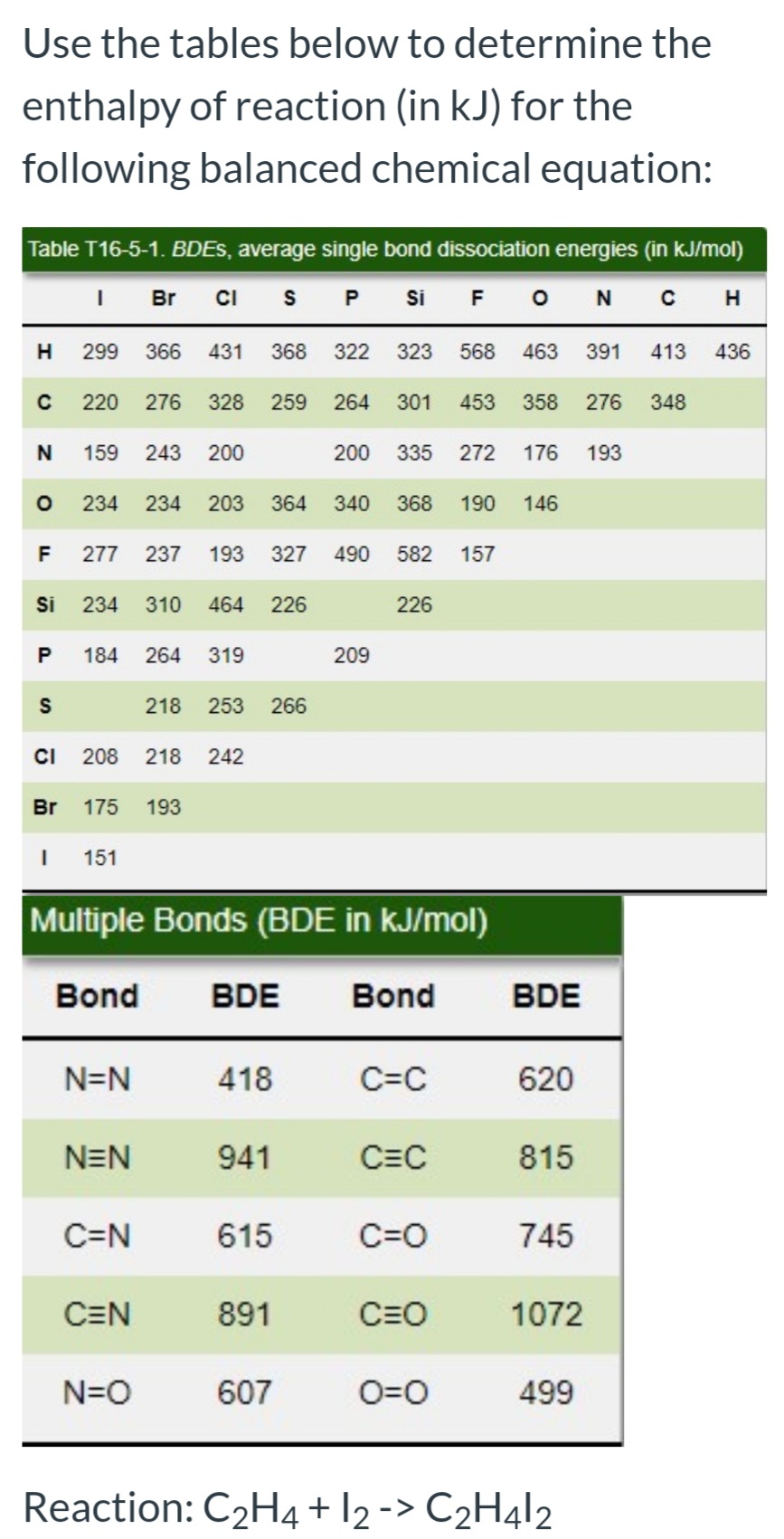
Use the tables below to determine the enthalpy of reaction (in kJ) for the following balanced chemical equation: Table T16-5-1. BDES, average single bond dissociation energies (in kJ/mol) I Br CI s P si F ONC H 299 366 431 368 322 323 568 463 391 413 436 220 276 328 259 264 301 453 358 276 348 N 159 243 200 200 335 272 176 193 234 234 203 364 340 368 190 146 F 277 237 193 327 490 582 157 Si 234 310 464 226 226 184 264 319 209 218 253 266 CI 208 218 242 Br 175 193 I 151 Multiple Bonds (BDE in kJ/mol) Bond BDE Bond BDE N=N 418 C=C 620 NEN 941 C=C 815 C=N 615 C=0 745 CEN 891 C=O 1072 N=O 607 O=0 499 Reaction: C2H4 + l2 -> C2H412 P.
Thermochemistry
Thermochemistry can be considered as a branch of thermodynamics that deals with the connections between warmth, work, and various types of energy, formed because of different synthetic and actual cycles. Thermochemistry describes the energy changes that occur as a result of reactions or chemical changes in a substance.
Exergonic Reaction
The term exergonic is derived from the Greek word in which ‘ergon’ means work and exergonic means ‘work outside’. Exergonic reactions releases work energy. Exergonic reactions are different from exothermic reactions, the one that releases only heat energy during the course of the reaction. So, exothermic reaction is one type of exergonic reaction. Exergonic reaction releases work energy in different forms like heat, light or sound. For example, a glow stick releases light making that an exergonic reaction and not an exothermic reaction since no heat is released. Even endothermic reactions at very high temperature are exergonic.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images








