What is AHn for the following chemical reaction? CO(g) + NH3(g)-HCN(g)+ H2O(g) You can use the following table of standard heats of formation (AH;) to calculate the enthalpy of the given reaction. Standard Heat of Formation (kJ/mol) Standard Heat of Element/ Compound Element/ Compound Formation (kJ/mol) H(g) 218 N(g) 473 H2(g) O2(g) 0. NH3(g) -45.90 O(g) 249 CO(g) -110.5 H2O(g) -241.8kJ C(g) 71 HCN(g) 130.5kJ C(s) HNO3 (aq) -206.6 Express the standard enthalpy of reaction to three significant figures and include the appropriate units.
What is AHn for the following chemical reaction? CO(g) + NH3(g)-HCN(g)+ H2O(g) You can use the following table of standard heats of formation (AH;) to calculate the enthalpy of the given reaction. Standard Heat of Formation (kJ/mol) Standard Heat of Element/ Compound Element/ Compound Formation (kJ/mol) H(g) 218 N(g) 473 H2(g) O2(g) 0. NH3(g) -45.90 O(g) 249 CO(g) -110.5 H2O(g) -241.8kJ C(g) 71 HCN(g) 130.5kJ C(s) HNO3 (aq) -206.6 Express the standard enthalpy of reaction to three significant figures and include the appropriate units.
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter12: Thermodynamic Processes And Thermochemistry
Section: Chapter Questions
Problem 75AP
Related questions
Question
100%
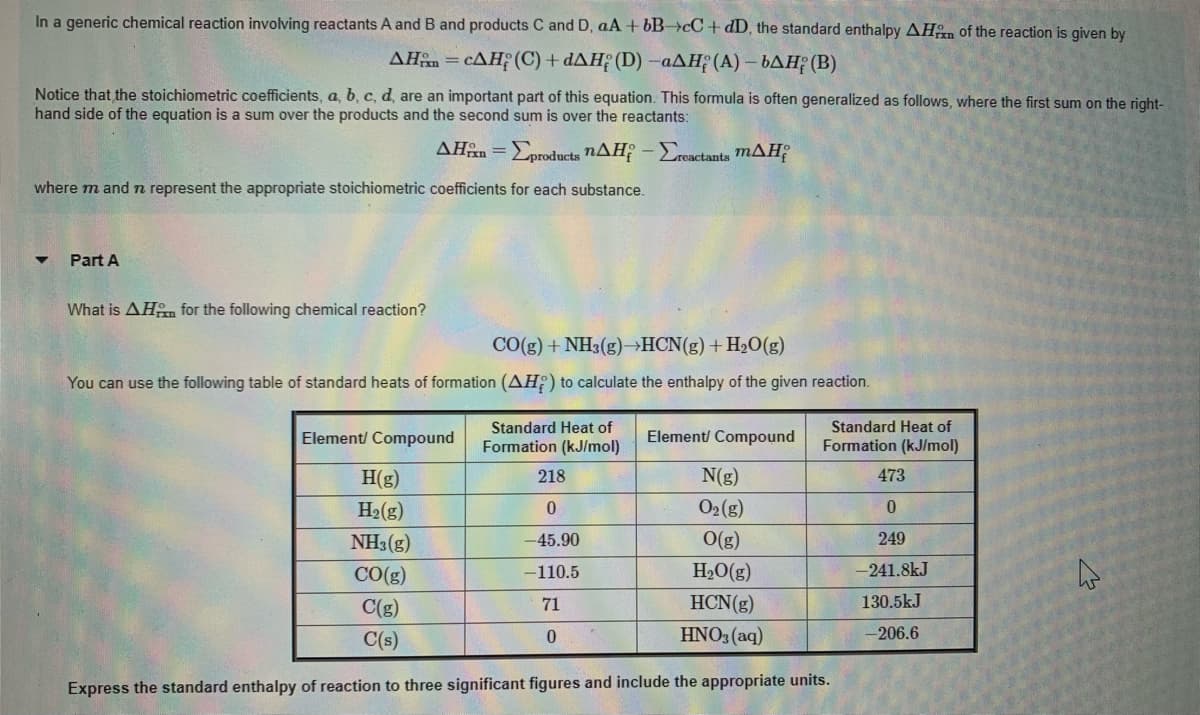
Transcribed Image Text:In a generic chemical reaction involving reactants A and B and products C and D, aA + bB>cC+dD, the standard enthalpy AHen of the reaction is given by
AHan = cAH; (C) + dAH; (D) -aAH; (A) – bAH¡ (B)
Notice that the stoichiometric coefficients, a, b, c, d, are an important part of this equation. This formula is often generalized as follows, where the first sum on the right-
hand side of the equation is a sum over the products and the second sum is over the reactants:
AHn = Eproducts nAH - Ereactants mAH;
where m and n represent the appropriate stoichiometric coefficients for each substance.
Part A
What is AHm for the following chemical reaction?
CO(g) + NH3(g)→HCN(g)+H2O(g)
You can use the following table of standard heats of formation (AH) to calculate the enthalpy of the given reaction.
Standard Heat of
Formation (kJ/mol)
Standard Heat of
Element/ Compound
Element/ Compound
Formation (kJ/mol)
H(g)
218
N(g)
473
H2(g)
O2 (g)
NH3 (g)
-45.90
O(g)
249
H2O(g)
HCN(g)
CO(g)
-110.5
-241.8kJ
C(g)
71
130.5kJ
C(s)
HNO3 (aq)
-206.6
Express the standard enthalpy of reaction to three significant figures and include the appropriate units.

Transcribed Image Text:Standard Enthalpy of Reaction
Learning Goal:
To understand how standard enthalpy of reaction is related
to the standard heats of formation of the reactants and
products.
The standard enthalpy of reaction is the enthalpy change
that occurs in a reaction when
are in their standard states. The symbol for the standard
enthalpy of reaction is AHn, where the subscript "rxn"
stands for "reaction." The standard enthalpy of a reaction is
calculated from the standard heats of formation (AHº)
(subscript "f" for formation) of its reactants and products.
Therefore, the standard enthalpy AH of any reaction can
be mathematically determined, as long as the standard heats
of formation (AH;)of its reactants and products are known.
the reactants and products
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning