Use the van der Waals equation of state to calculate the pressure of 3.20 mol of CH, at 479 K in a 5.60 L vessel. Van der Waals constants can be found in the van der Waals constants table. P = atm Use the ideal gas equation to calculate the pressure under the same conditions. P = atm Under these conditions, would you expect CH, or CCl, to deviate more from ideal behavior? Why? O CCI, because it occupies a larger volume and it has greater dispersion forces between molecules. CCI, because it occupies a smaller volume and it has greater dispersion forces between molecules. CH, because it occupies a smaller volume and it has greater dispersion forces between molecules. CH, because it occupies a smaller volume and it has smaller dispersion forces between molecules. CH, because it occupies a larger volume and it has smaller dispersion forces between molecules. CCI because it occupies a larger volume and it has smaller dispersion forces between molecules.
Use the van der Waals equation of state to calculate the pressure of 3.20 mol of CH, at 479 K in a 5.60 L vessel. Van der Waals constants can be found in the van der Waals constants table. P = atm Use the ideal gas equation to calculate the pressure under the same conditions. P = atm Under these conditions, would you expect CH, or CCl, to deviate more from ideal behavior? Why? O CCI, because it occupies a larger volume and it has greater dispersion forces between molecules. CCI, because it occupies a smaller volume and it has greater dispersion forces between molecules. CH, because it occupies a smaller volume and it has greater dispersion forces between molecules. CH, because it occupies a smaller volume and it has smaller dispersion forces between molecules. CH, because it occupies a larger volume and it has smaller dispersion forces between molecules. CCI because it occupies a larger volume and it has smaller dispersion forces between molecules.
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.103QP: Calculate the molar volume of ethane at 1.00 atm and 0C and at 10.0 atm and 0C, using the van der...
Related questions
Question
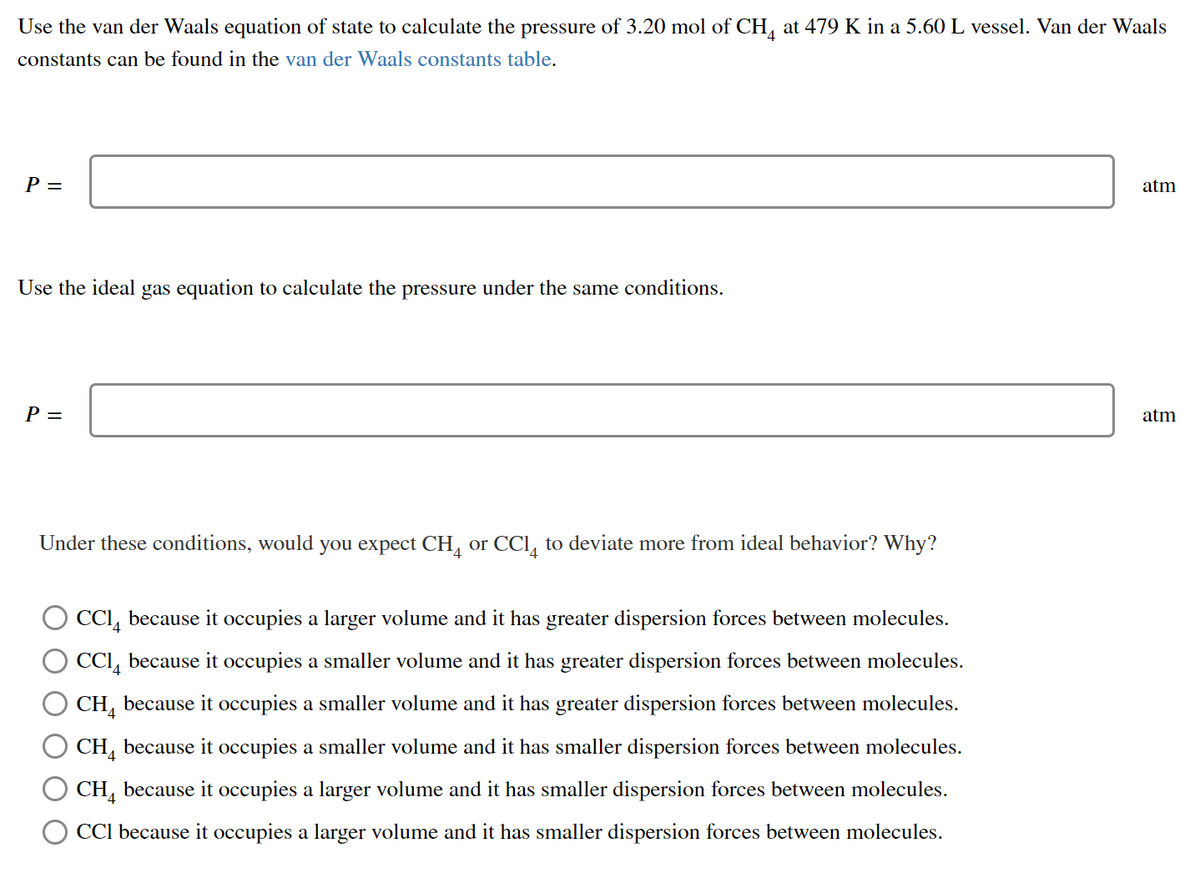
Transcribed Image Text:Use the van der Waals equation of state to calculate the pressure of 3.20 mol of CH, at 479 K in a 5.60L vessel. Van der Waals
constants can be found in the van der Waals constants table.
P =
atm
Use the ideal gas equation to calculate the pressure under the same conditions.
P =
atm
Under these conditions, would you expect CH, or CCl, to deviate more from ideal behavior? Why?
CCI, because it occupies a larger volume and it has greater dispersion forces between molecules.
CCI, because it occupies a smaller volume and it has greater dispersion forces between molecules.
CH, because it occupies a smaller volume and it has greater dispersion forces between molecules.
CH, because it occupies a smaller volume and it has smaller dispersion forces between molecules.
CH, because it occupies a larger volume and it has smaller dispersion forces between molecules.
CCl because it occupies a larger volume and it has smaller dispersion forces between molecules.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning