Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter20: Chemistry Of The Metals
Section: Chapter Questions
Problem 34QAP: The equilibrium constant for the reaction 2CrO42(aq)+2H+(aq)Cr2O72(aq)+H2O is 31014 . What must the...
Related questions
Question
Using heats of formation given, calculate the heat of reaction for the following reaction:
Fe2O3(s) + 2Al(s) → Al2O3(s) + 2Fe(s)
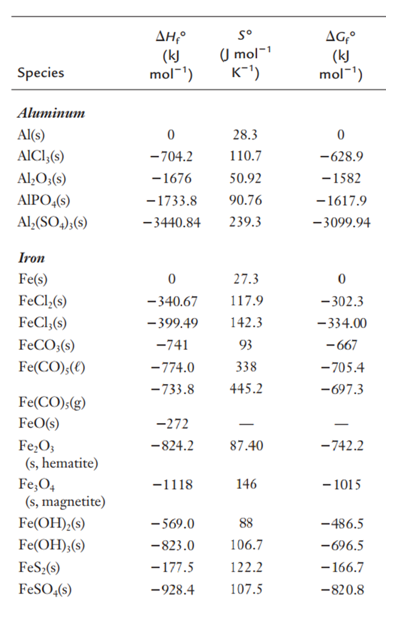
Transcribed Image Text:AH,°
(k)
mol"')
U mol-1
K"')
AG;°
(kJ
mol-')
Species
Aluminum
Al(s)
28.3
AICI,(s)
- 704.2
110.7
-628.9
Al,O;(s)
-1676
50.92
-1582
AIPO,(s)
-1733.8
90.76
-1617.9
Al,(SO,),(s)
-3440.84
239.3
-3099.94
Iron
Fe(s)
27.3
FeCl,(s)
-340.67
117.9
- 302.3
FeCl;(s)
- 399.49
142.3
-334.00
FECO3(s)
-741
93
- 667
Fe(CO),(€)
-774.0
338
-705.4
-733.8
445.2
-697.3
Fe(CO)s(g)
FeO(s)
-272
-
Fe,O;
-824.2
87.40
-742.2
(s, hematite)
- 1015
Fe,O,
(s, magnetite)
Fe(OH),(s)
-1118
146
- 569.0
88
-486.5
Fe(OH),(s)
-823.0
106.7
-696.5
FeS;(s)
-177.5
122.2
- 166.7
FESO,(s)
-928.4
107.5
-820.8
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning



Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

