Using the diagram in Figure 3, predict the following for the distillation of a solution of x and y which has an initial concentration of 70% x and 30% y. a. Initial boiling point b. Vapor composition at initial boiling point Suppose that gas chromatographic analysis of a solution of A and B gave peaks with the following measurements: A, height - 180 mm, width at half-height - 4 mm; B, height - 64 mm, width at half-height - 7 mm. What is the composition (weight %) of the solution?
Using the diagram in Figure 3, predict the following for the distillation of a solution of x and y which has an initial concentration of 70% x and 30% y. a. Initial boiling point b. Vapor composition at initial boiling point Suppose that gas chromatographic analysis of a solution of A and B gave peaks with the following measurements: A, height - 180 mm, width at half-height - 4 mm; B, height - 64 mm, width at half-height - 7 mm. What is the composition (weight %) of the solution?
Chapter32: Gas Chromatography
Section: Chapter Questions
Problem 32.15QAP
Related questions
Question
Using the diagram in Figure 3, predict the following for the distillation of a solution of x and y which has an initial concentration of 70% x and 30% y.
a. Initial boiling point
b. Vapor composition at initial boiling point
Suppose that gas chromatographic analysis of a solution of A and B gave peaks with the following measurements: A, height - 180 mm, width at half-height - 4 mm; B, height - 64 mm, width at half-height - 7 mm. What is the composition (weight %) of the solution?
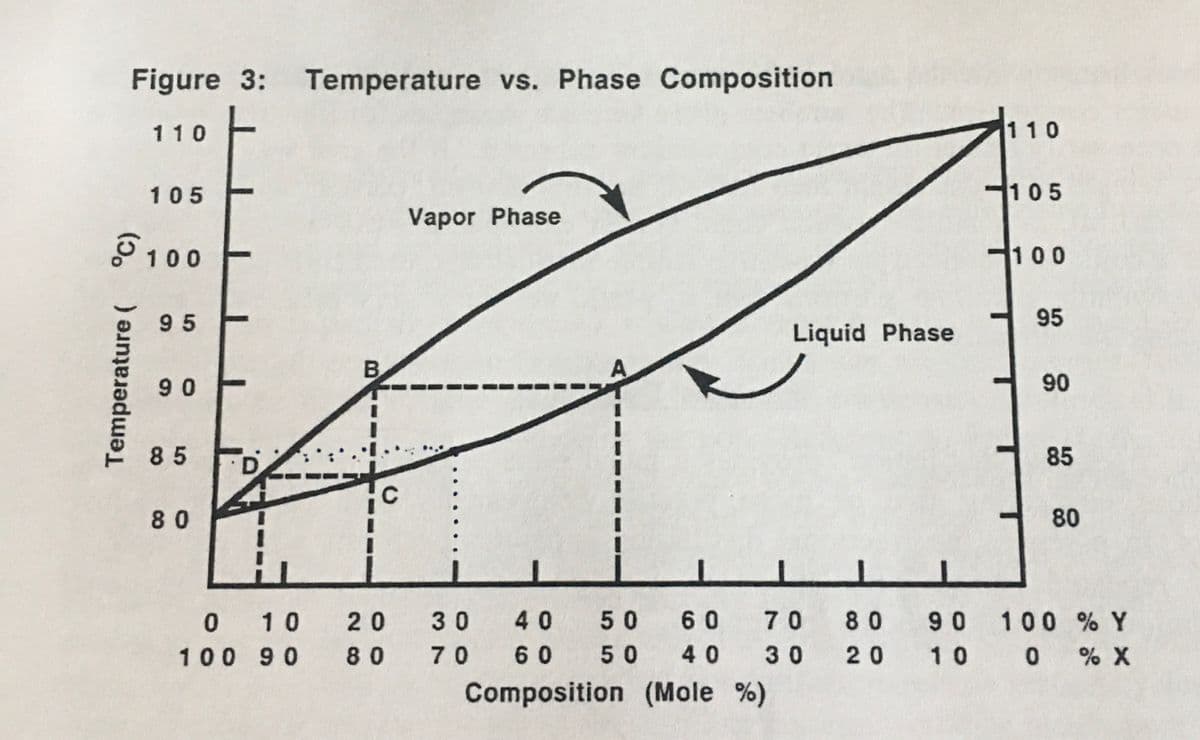
Transcribed Image Text:Figure 3: Temperature vs. Phase Composition.
110
105
81
Temperature (
100
95
90
85
80
B
0
100 90
10 20
80
Vapor Phase
Liquid Phase
50 60 70 80
30 40
70 60 50 40 30 20
Composition (Mole %)
110
105
100
95
90
85
80
90 100
10
% Y
0
0 % X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT