Using the equation below, convert 17.19 grams of water to moles of H2SO4. Please round your answer to two digits after the decimal point and don't forget units and substance! SO3 + H2O --> H2SO4
Using the equation below, convert 17.19 grams of water to moles of H2SO4. Please round your answer to two digits after the decimal point and don't forget units and substance! SO3 + H2O --> H2SO4
Chapter5: Chemical Reactions
Section: Chapter Questions
Problem 5.40E
Related questions
Question
Using the equation below, convert 17.19 grams of water to moles of H2SO4. Please round your answer to two digits after the decimal point and don't forget units and substance!
SO3 + H2O --> H2SO4
Expert Solution
Step 1
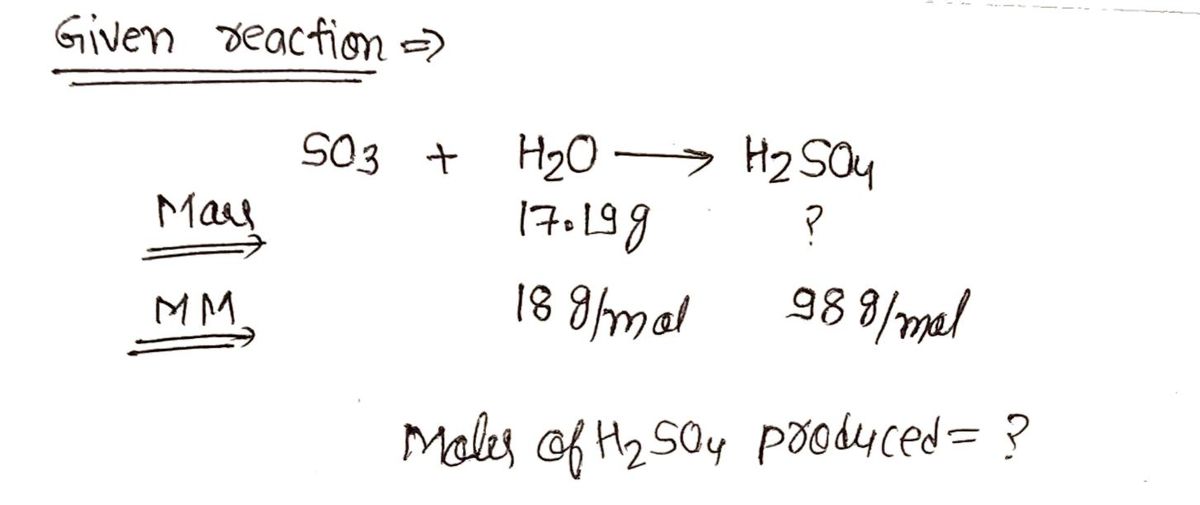
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
