Using the following equation: Pb(SO4)2 + 4LINO3 --> Pb(NO3)4 + 2Lİ2SO4 How many grams of lithium nitrate will be needed to make 250.0 grams of lithium sulfate, assuming that you have an adequate amount of lead(IV) sulfate to do the reaction? Round your final answer to 1 place after the decimal. Answer: grams lithium nitrate
Using the following equation: Pb(SO4)2 + 4LINO3 --> Pb(NO3)4 + 2Lİ2SO4 How many grams of lithium nitrate will be needed to make 250.0 grams of lithium sulfate, assuming that you have an adequate amount of lead(IV) sulfate to do the reaction? Round your final answer to 1 place after the decimal. Answer: grams lithium nitrate
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter21: The Chemistry Of The Main Group Elements
Section: Chapter Questions
Problem 107GQ: A Boron and hydrogen form an extensive family of compounds, and the diagram below shows how they are...
Related questions
Question
Transcribed Image Text:7.5 Stoichiometry - concept check
Started: Oct 2 at 10:37am
Quiz Instructions
Stoichiometry problems take practice! Get started here.
Return to the Mole-Mass and Mass-Mass Problems problems
Question 2
1 pts
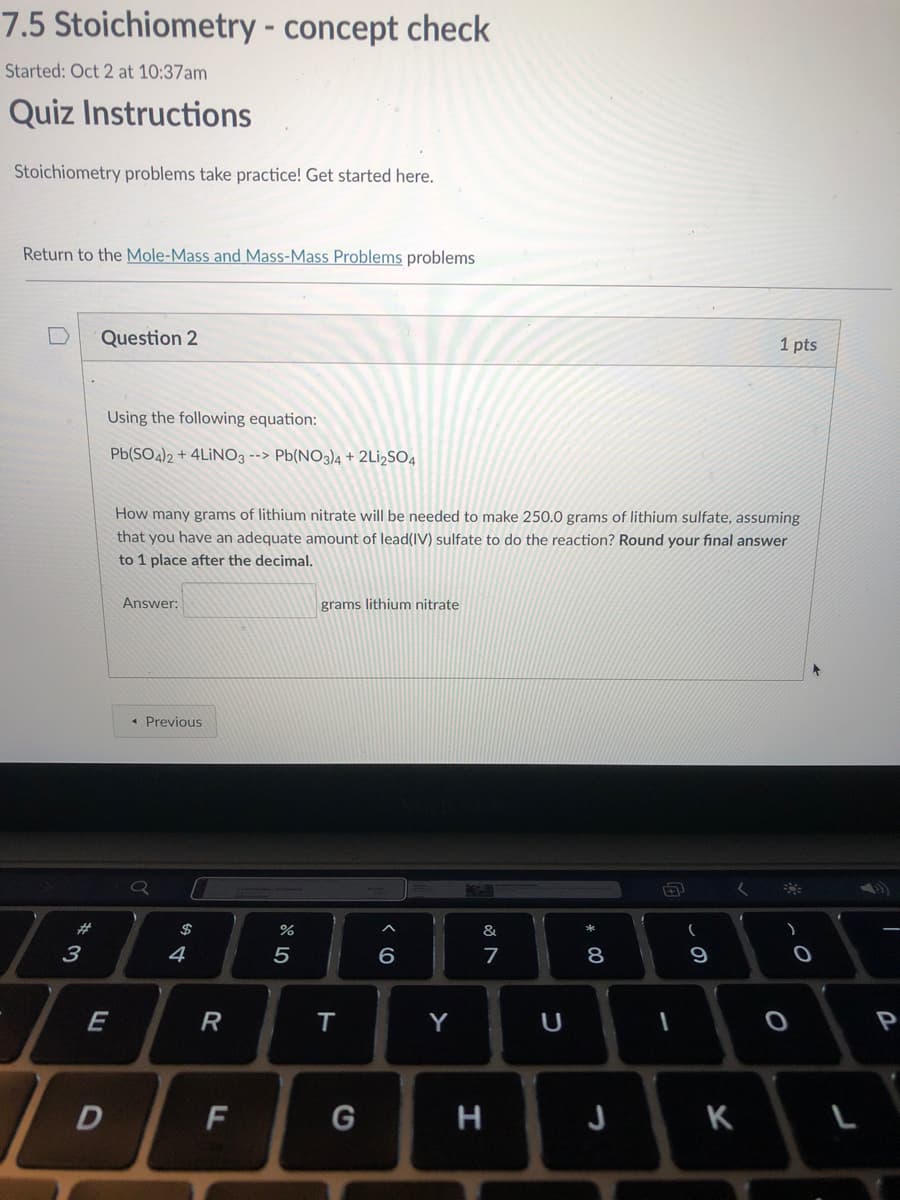
Using the following equation:
Pb(SO4)2 + 4LİNO3 --> Pb(NO3)4 + 2L¡2SO4
How many grams of lithium nitrate will be needed to make 250.0 grams of lithium sulfate, assuming
that you have an adequate amount of lead(IV) sulfate to do the reaction? Round your final answer
to 1 place after the decimal.
Answer:
grams lithium nitrate
• Previous
$
&
3
4
6
9
E
Y
U
P
F
G
H
K L
* CO
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning