Using the following figure, predict the boiling point of the initial drops of distillate of a mixture of .8 Mol fraction of toluene and .2 Mol fraction of cyclohexane.
Using the following figure, predict the boiling point of the initial drops of distillate of a mixture of .8 Mol fraction of toluene and .2 Mol fraction of cyclohexane.
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter6: Equilibria In Single-component Systems
Section: Chapter Questions
Problem 6.3E
Related questions
Question
100%
Using the following figure, predict the boiling point of the initial drops of distillate of a mixture of .8 Mol fraction of toluene and .2 Mol fraction of cyclohexane.
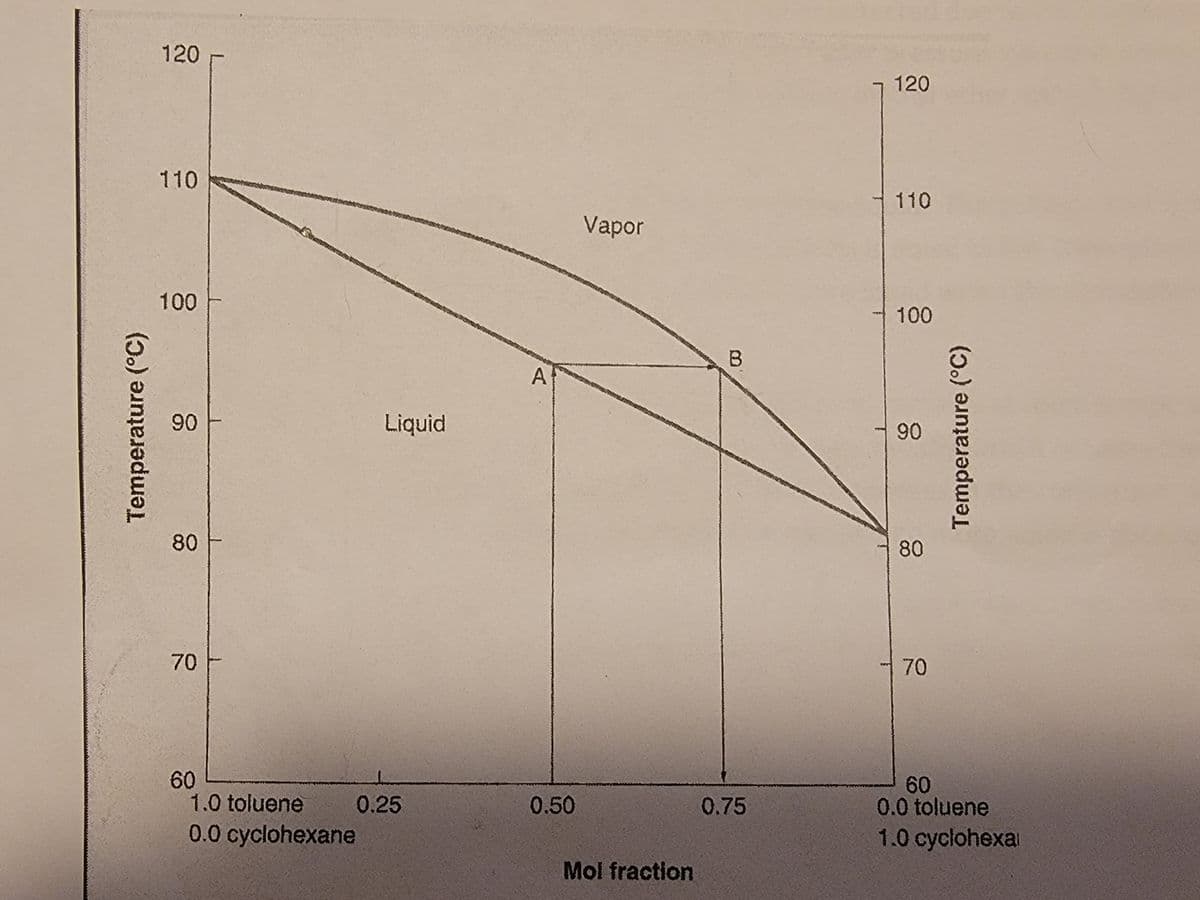
Transcribed Image Text:120
120
110
110
Vapor
100
100
A
90
Liquid
90
80
70
70
60
1.0 toluene
60
0.0 toluene
0.25
0.50
0.75
0.0 cyclohexane
1.0 cyclohexal
Mol fraction
Temperature (C)
80
Temperature (°C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole