Using the information given answer the following 3 questions. Thank you. Calculate the molar concentration of sodium hydroxide in the solution (mol/L). Using the equation for the reaction, determine the number of moles of sodium hydroxide that reacted.
Determine the number of moles of hydrochloric acid that reacted.
Using the information given answer the following 3 questions. Thank you. Calculate the molar concentration of sodium hydroxide in the solution (mol/L). Using the equation for the reaction, determine the number of moles of sodium hydroxide that reacted. Determine the number of moles of hydrochloric acid that reacted.
Chapter7: Neutralization Titrations And Graphical Representations
Section: Chapter Questions
Problem 13P
Related questions
Question
Using the information given answer the following 3 questions. Thank you.
Calculate the molar concentration of sodium hydroxide in the solution (mol/L).
Using the equation for the reaction, determine the number of moles of sodium hydroxide that reacted.
Determine the number of moles of hydrochloric acid that reacted.

Transcribed Image Text:Lab 4: Quantitative Titration
Concentration of HCl used:
23.00mL
Volume of HCl used:
fhenalphthalein
fink lClear
Indicator:
Endpoint colour change:
Table 1: Titration of Sodium Hydroxide with standard Hydrochloric Acid.
Burette reading (mL)
Trial 1
Trial 2
Trial 3
Trial 4
Final reading
Initial reading
Volume of NaOH(aq)
(if required)
22:30
O-00ML 0.0omL
22.7 6m22.51m 2250
Average of 2 closest values to within 0.10 mL: 591md
2341mL
O.ComL
22.76mL 2257
0.00ML
used
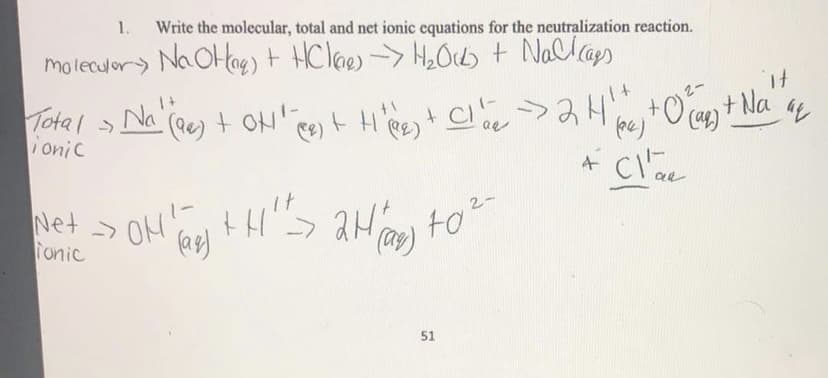
Transcribed Image Text:1.
Write the molecular, total and net ionic equations for the neutralization reaction.
moleculor) Na OHag) t HClaes -> H2Ocb + Nallaps
Total > t H'Re+ C
Na (ee) + OH' ee)
t.
Ca) +Na
onic
Net ->
Tonic
ON
to2-
51
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

