Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter19: Transition Metals And Coordination Chemistry
Section: Chapter Questions
Problem 17E: Predict the products of each of the following reactions. (Note: In addition to using the information...
Related questions
Question
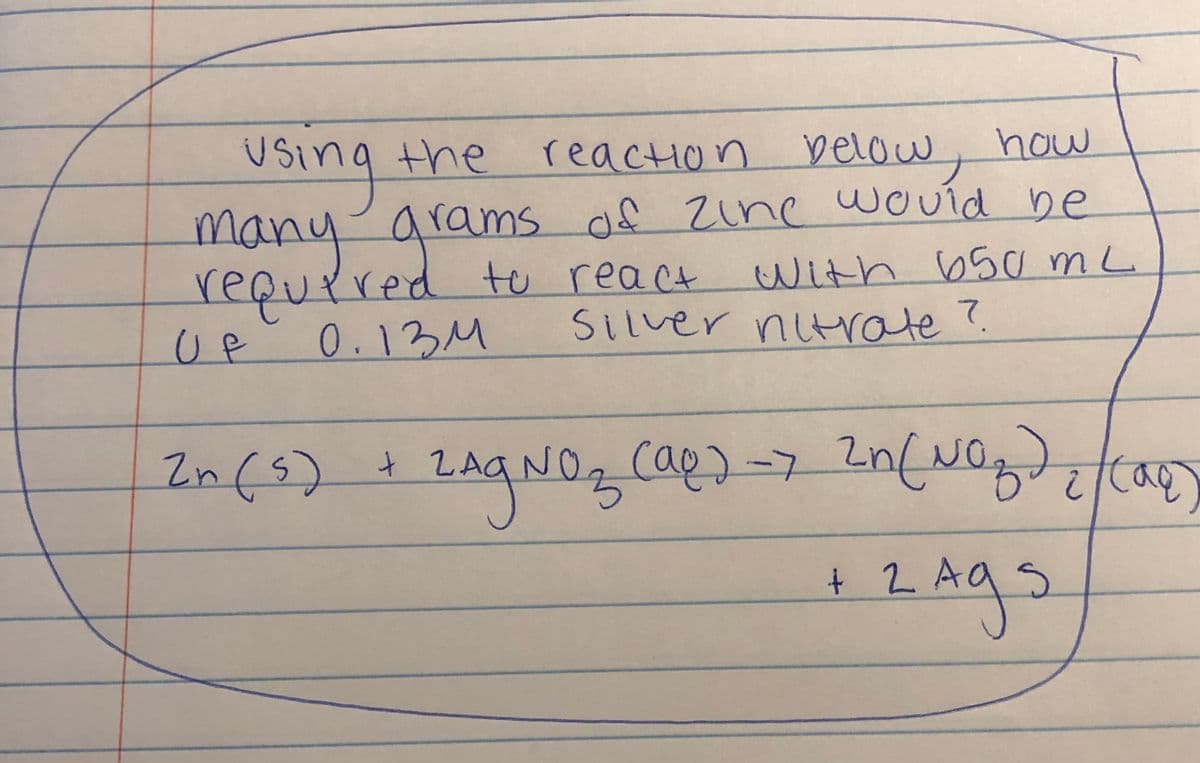
Transcribed Image Text:reaction below, how
of Zine would be
With 650 mL
Using the
USino
the
arams of zine wcVíd be
many
requrred te react
0.13M
Silver nitrate?.
UP
In(s)
cap)-7
NO.
+ 2.
2Ags
Expert Solution
Given
In the given question, the reaction happening in the solution mixture is given as-
The concentration of the silver nitrate solution = 0.13 M
The volume of silver nitrate solution = 650 mL = 0.65 L
Molar mass of silver nitrate = 169.87 g/mol
Molar mass of zinc = 65.38 g/mol
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning