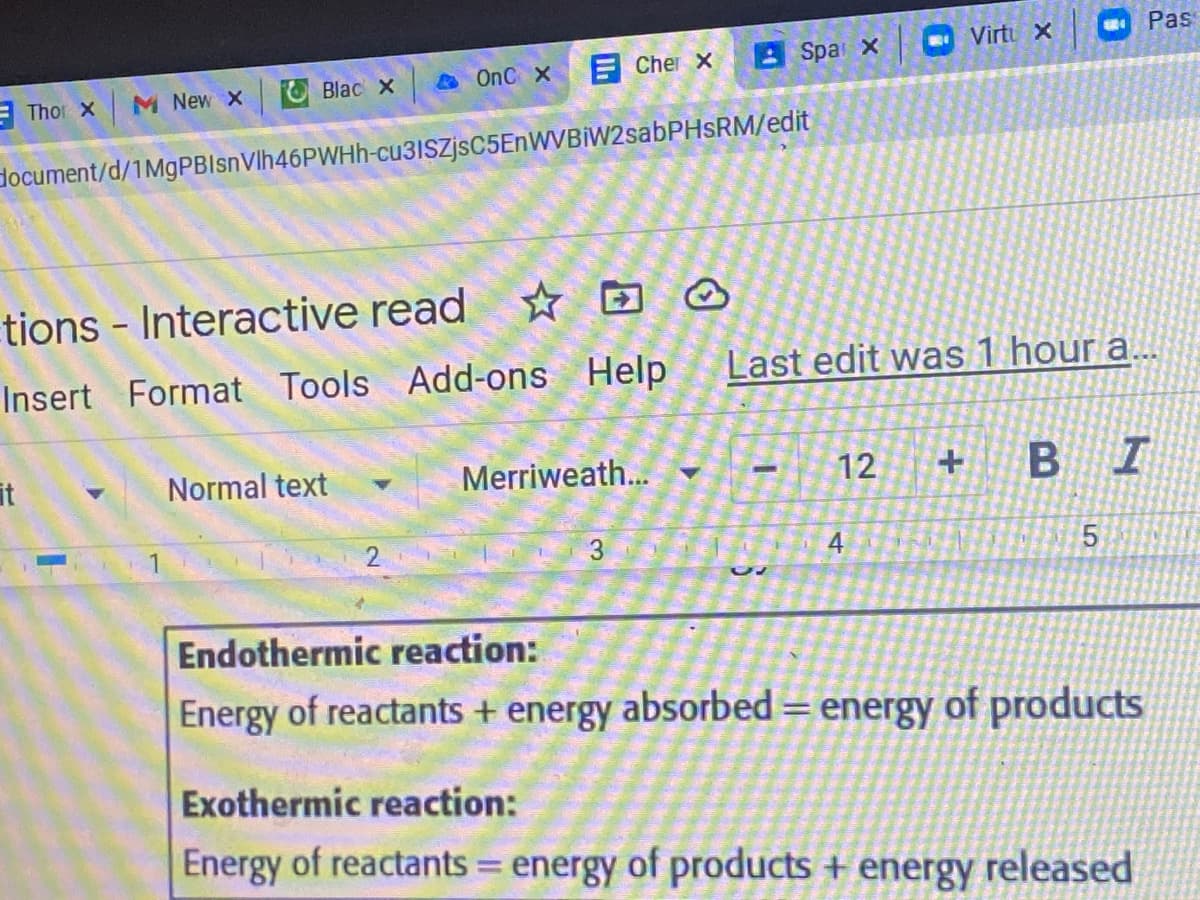
Using the model below answer the question. Explain: How can products and reactants have different amounts of energy without violating the law of conversation of energy?
Using the model below answer the question. Explain: How can products and reactants have different amounts of energy without violating the law of conversation of energy?
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter20: Molecular Mass Spectrometry
Section: Chapter Questions
Problem 20.16QAP
Related questions
Question
Using the model below answer the question. Explain: How can products and reactants have different amounts of energy without violating the law of conversation of energy?
Transcribed Image Text:Pas
Virtu X
E Cher X
A Spa x
E Thor x
M New X
OBlac x
&OnC X
document/d/1MGPBISNVIH46PWHH-cu3ISzjsc5EnWVBIW2sabPHsRM/edit
tions - Interactive read D O
Insert Format Tools
Add-ons
Help
Last edit was 1 hour a..
it
Normal text
Merriweath...
12
BI
4
5.
Endothermic reaction:
Energy of reactants + energy absorbed = energy of products
Exothermic reaction:
Energy of reactants = energy of products + energy released
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax